推荐产品
化驗
98%
mp
145-148 °C (lit.)
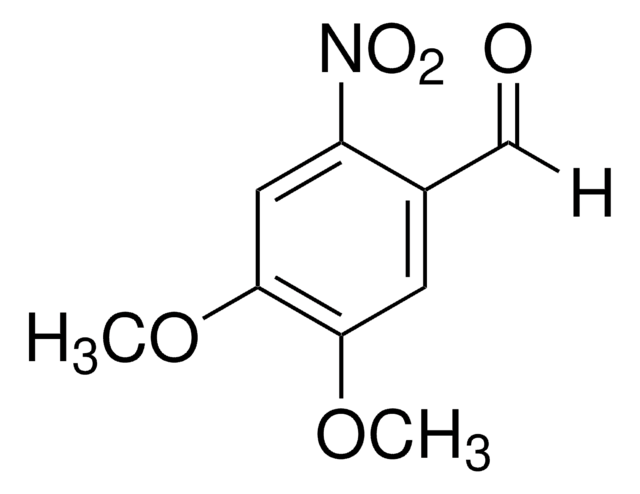
SMILES 字串
COc1cc(CO)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H11NO5/c1-14-8-3-6(5-11)7(10(12)13)4-9(8)15-2/h3-4,11H,5H2,1-2H3
InChI 密鑰
WBSCOJBVYHQOFB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4,5-二甲氧基-2-硝基苄醇(6-硝基藜芦醇)是2-硝基苄醇的衍生物。据报道,它是通过木质素过氧化物酶(分离自黄孢原毛平革菌)催化的,藜芦醇(3,4-二甲氧基苄基)醇的氧化产物之一。
應用
4,5-二甲氧基-2-硝基苄醇(6-硝基藜芦醇)适用于合成4,5-二甲氧基-2-硝基苄基甲基丙烯酸酯(一种对光不稳定的单体) 和2-(4-((4-(4,5-二甲氧基-2-硝基苄氧基)苯基)亚环己基)甲基)苯氧基)-N,N-二甲基乙胺(一种笼状环芬-OH配体)。
它可用于合成以下物质:
它可用于合成以下物质:
- 1-[[((氯羰基)氧基]甲基]-4,5-二甲氧基-2-硝基苯
- 双(4,5-二甲氧基-2-硝基苯基)乙二醇,一种对光不稳定的保护基
- 光学敏感单体
- 硝基藜芦(NV)保护的α-羟基乙酸 (αG) (NV-αG-OH),其是在硝基藜芦(NV)保护的α-羟基乙酸(α-G)的氰基甲基(CM)酯 (αG) (NV-αG-CM)的制备中所必须的
- 4,5-二甲氧基-2-硝基苄基-p-硝基碳酸苯酯
- 6-硝基藜芦氧羰基氯(NVOCCl),一种用于保护氨基糖中氨基功能的试剂
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Andrew A Brown et al.
Langmuir : the ACS journal of surfaces and colloids, 25(3), 1744-1749 (2009-01-10)
The use of photolabile protecting groups (PGs) as a means to create latent hydrophilic surfaces is presented. Naturally hydrophobic PGs, based on o-nitrobenzyl chemistry, are used on polymer side chains, poised for cleavage upon exposure to UV light. Removal of
Nadezda Fomina et al.
Journal of the American Chemical Society, 132(28), 9540-9542 (2010-06-24)
A new light-sensitive polymer containing multiple light-sensitive triggering groups along the backbone and incorporating a quinone-methide self-immolative moiety was developed and formulated into nanoparticles encapsulating a model pharmaceutical Nile Red. Triggered burst release of the payload upon irradiation and subsequent
Bis (4, 5-dimethoxy-2-nitrophenyl) ethylene glycol: a new and efficient photolabile protecting group for aldehydes and ketones.
Kantevari S, et al.
Tetrahedron, 61(24), 5849-5854 (2005)
Photosensitive protecting groups of amino sugars and their use in glycoside synthesis. 2-nitrobenzyloxycarbonylamino and 6-nitroveratryloxycarbonylamino derivatives.
Amit B, et al.
The Journal of Organic Chemistry, 39(2), 192-196 (1974)
Deepak Kumar Sinha et al.
Chembiochem : a European journal of chemical biology, 11(5), 653-663 (2010-02-27)
We have implemented a noninvasive optical method for the fast control of protein activity in a live zebrafish embryo. It relies on releasing a protein fused to a modified estrogen receptor ligand binding domain from its complex with cytoplasmic chaperones
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门