推荐产品
化驗
99%
光學活性
[α]20/D −157°, c = 1 in pyridine
光學純度
ee: 99% (HPLC)
mp
242-244 °C (lit.)
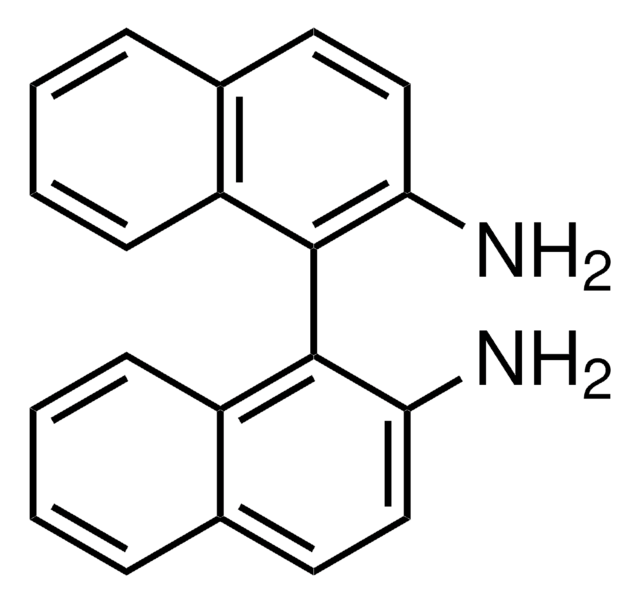
SMILES 字串
Nc1ccc2ccccc2c1-c3c(N)ccc4ccccc34
InChI
1S/C20H16N2/c21-17-11-9-13-5-1-3-7-15(13)19(17)20-16-8-4-2-6-14(16)10-12-18(20)22/h1-12H,21-22H2
InChI 密鑰
DDAPSNKEOHDLKB-UHFFFAOYSA-N
應用
用于合成手性内酯。
(S)-(-)-1,1′-Binaphthyl-2,2′-diamine may be used in the synthesis of (S)-(+)-N,N′-dimethyl-N,N′-bis{3-[bis-(1-methyl-2-benzimidazolyl methyl)]-amino]-propyl}-1,1′-binaphthyl-2,2′-diamine, an octadentate ligand that can form dinuclear and trinuclear copper(II) complexes. It may also be used in the synthesis of (Sa)-N-[2´-amino-(1,1´-binaphthyl)-2-yl]-4-methylbenzenesulfonamide.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Models for biological trinuclear copper clusters. Characterization and enantioselective catalytic oxidation of catechols by the copper (II) complexes of a chiral ligand derived from (S)-(-)-1, 1'-binaphthyl-2, 2'-diamine.
Mimmi MC, et al.
Dalton Transactions, 14, 2192-2201 (2004)
The Journal of Organic Chemistry, 56, 1112-1112 (1991)
(Sa, S)-N-[2'-(4-Methylphenylsulfonamido)-1, 1'-Binaphthyl-2-Yl] Pyrrolidine-2-Carboxamide: An Organocatalyst for the Direct Aldol Reaction.
Vizoquez SF, et al.
Organic Syntheses, 317-329 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门