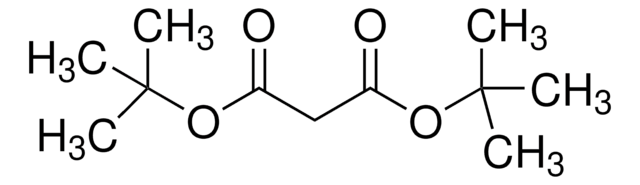
推荐产品
化驗
96%
形狀
liquid
折射率
n20/D 1.425 (lit.)
bp
102-104 °C/11 mmHg (lit.)
密度
1.014 g/mL at 25 °C (lit.)
SMILES 字串
CCOC(=O)C(C(=O)OCC)C(C)(C)C
InChI
1S/C11H20O4/c1-6-14-9(12)8(11(3,4)5)10(13)15-7-2/h8H,6-7H2,1-5H3
InChI 密鑰
RJNICNBRGVKNSR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Diethyl tert-butylmalonate has been used in the preparation of enantiomers of 4-tert-butyl-3-isopropyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane 1-sulfide (TBIPPS).
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
199.4 °F - closed cup
閃點(°C)
93 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
X L Ju et al.
Bioorganic & medicinal chemistry, 8(9), 2337-2341 (2000-10-12)
The enantiomers of 4-tert-butyl-3-isopropyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2 ]octane 1-sulfide (TBIPPS) were prepared in nine steps from diethyl tert-butylmalonate, and their abilities to compete with [3H]1-(4-ethynylphenyl)-4-n-propyl-2,6,7-trioxabicyclo[2.2.2 ]octane (EBOB), a noncompetitive antagonist of ionotropic gamma-aminobutyric acid (GABA) receptors, at their binding site were investigated using
Grazia Luisi et al.
Antioxidants (Basel, Switzerland), 8(1) (2018-12-28)
Oxidative damage is among the factors associated with the onset of chronic pathologies, such as neurodegenerative and metabolic diseases. Several classes of anti-oxidant compounds have been suggested as having a protective role against cellular stressors, but, in this perspective, peptides'
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门