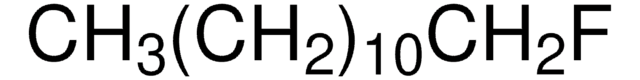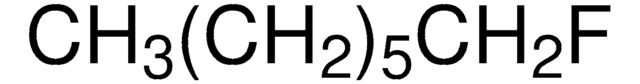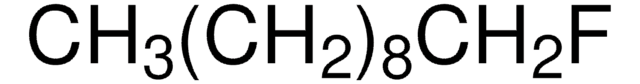推荐产品
化驗
98%
形狀
liquid
折射率
n20/D 1.396 (lit.)
bp
142-146 °C (lit.)
密度
0.814 g/mL at 25 °C (lit.)
官能基
alkyl halide
SMILES 字串
CCCCCCCCF
InChI
1S/C8H17F/c1-2-3-4-5-6-7-8-9/h2-8H2,1H3
InChI 密鑰
DHIVLKMGKIZOHF-UHFFFAOYSA-N
一般說明
1-Fluorooctane undergoes C-F bond-cleavage reaction with phenyl magnesium chloride to give n-octylbenzene. It reacts rapidly with trimethylsilyl iodide to give corresponding octyl iodides and trimethylsilyl fluoride.
應用
1-Fluorooctane has been used as a molecular probe in evaluations of gas chromatographic stationary phases consisting of bromo- and chloro-derivatives of a C78 branched alkane.
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
107.6 °F - closed cup
閃點(°C)
42 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Kouki Matsubara et al.
Organic letters, 11(8), 1765-1768 (2009-03-14)
An unexpected C-F bond-cleavage reaction of unactivated fluoroalkanes with the well-known Grignard reagents without using metal catalysts has been discovered. For example, a reaction between 1-fluorooctane and phenyl magnesium chloride gave n-octylbenzene in moderate yield. This coupling reaction via the
Ervin Sz Kováts et al.
Journal of chromatography. A, 1113(1-2), 206-219 (2006-02-25)
In a paper published in 1992 [K.S. Reddy, J.-Cl. Dutoit, E.sz. Kováts, Pair-wise interactions by gas chromatography. I. Interaction free enthalpies of solutes with non-associated primary alcohol groups, J. Chromatogr. 609 (1992) 229] retention indices and standard chemical potential differences
Halogen redistribution reactions between alkyl halides and trimethylsilyl iodide.
Friedrich EC and De Lucca G.
Journal of Organometallic Chemistry, 226(2), 143-148 (1982)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门