推荐产品
化驗
98%
形狀
liquid
折射率
n20/D 1.505 (lit.)
bp
90 °C/21 mmHg (lit.)
密度
1.116 g/mL at 25 °C (lit.)
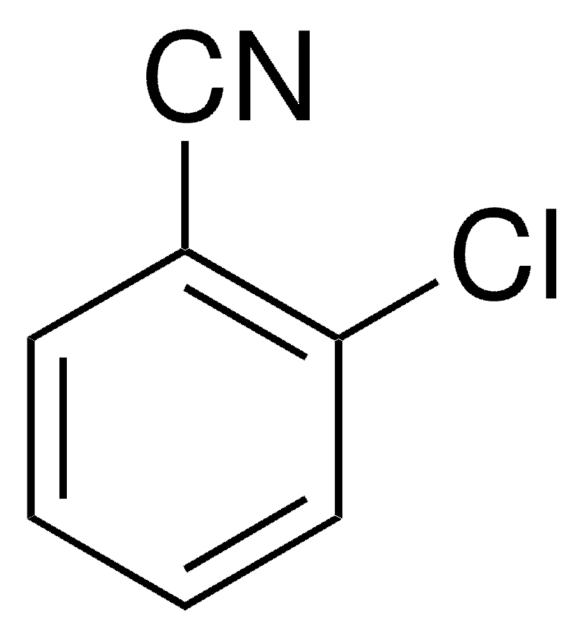
SMILES 字串
Fc1ccccc1C#N
InChI
1S/C7H4FN/c8-7-4-2-1-3-6(7)5-9/h1-4H
InChI 密鑰
GDHXJNRAJRCGMX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-Fluorobenzonitrile reacts with lithium N,N-dialkylaminoborohydride reagent to yield 2-(N,N-dialkylamino)benzylamines.
應用
2-Fluorobenzonitrile was used in the synthesis of :
- 3-amino-1,2-benzisoxazoles
- 6-(acetylaminomethyl)-3-amino-1,2-benzisoxazole
- 5-(4′-methyl [1, 1′-biphenyl]-2-yl)-1H-tetrazole
- xanthone-iminium triflates
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
165.2 °F - closed cup
閃點(°C)
74 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
H M Colquhoun et al.
Organic letters, 3(15), 2337-2340 (2001-07-21)
[reaction: see text] Condensation of 2-fluorobenzonitriles with phenoxides affords 2-aryloxybenzonitriles that cyclize cleanly in trifluoromethanesulfonic acid at room temperature to give xanthone-iminium triflates. The C=N bond in these compounds is remarkably resistant to hydrolysis, but prolonged reaction with strong aqueous
S Thomas et al.
The Journal of organic chemistry, 66(6), 1999-2004 (2001-04-13)
A novel tandem amination-reduction reaction has been developed in which 2-(N,N-dialkylamino)benzylamines are generated from 2-halobenzonitriles and lithium N,N-dialkylaminoborohydride (LAB) reagents. These reactions are believed to occur through a tandem S(N)Ar amination-reduction mechanism wherein the LAB reagent promotes halide displacement by
Edmund J Norris et al.
PLoS neglected tropical diseases, 14(9), e0008365-e0008365 (2020-09-09)
Insecticide resistance poses a significant threat to the control of arthropods that transmit disease agents. Nanoparticle carriers offer exciting opportunities to expand the armamentarium of insecticides available for public health and other pests. Most chemical insecticides are delivered by contact
S D Lepore et al.
The Journal of organic chemistry, 65(10), 2924-2932 (2000-05-18)
Further exploration of the scope of our solid-phase method for the synthesis of 3-aminobenzisoxazoles (using the Kaiser oxime resin 1) is described. The effects of base, leaving group, and solvent on the nucleophilic aromatic substitution based resin-loading reaction are discussed.
Efficient synthesis of 5-(4'-methyl [1, 1'-biphenyl]-2-yl)-1H-tetrazole.
Russell RK and Murray WV.
The Journal of Organic Chemistry, 58(18), 5023-5024 (1993)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门