195707
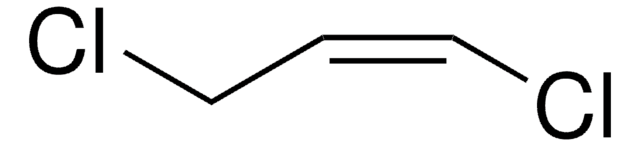
顺式-1,4-二氯-2-丁烯
95%
别名:
(2Z)-1,4-Dichloro-2-butene, (2Z)-1,4-Dichlorobut-2-ene, (Z)-1,4-Dichloro-2-butene, 1,4-Dichloro-cis-2-butene, cis-1,2-Bis(chloromethyl)ethene
登录查看公司和协议定价
所有图片(1)
About This Item
线性分子式:
ClCH2CH=CHCH2Cl
CAS号:
分子量:
125.00
Beilstein:
1719692
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.489 (lit.)
bp
152 °C/758 mmHg (lit.)
mp
−48 °C (lit.)
密度
1.188 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
ClC\C=C/CCl
InChI
1S/C4H6Cl2/c5-3-1-2-4-6/h1-2H,3-4H2/b2-1-
InChI 密鑰
FQDIANVAWVHZIR-UPHRSURJSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
cis-1,4-Dichloro-2-butene on reaction with sodium amide yields trans-1-chloro-1,3-butadiene. Mechanism of condensation of sulfone-activated methylene compounds with cis-1,4-dichloro-2-butene to yield cyclopentene has been investigated.
應用
cis-1,4-Dichloro-2-butene was used in the preparation of functionalized 3,5-disubstituted cyclopent-2-enones.
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
131.0 °F - closed cup
閃點(°C)
55 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Wei-Chieh Cheng et al.
The Journal of organic chemistry, 67(13), 4387-4391 (2002-06-22)
The preparation of functionalized 3,5-disubstituted cyclopent-2-enones via a solid-phase sulfone linker strategy is described. Polystyrene/divinylbenzene sulfinate 1 underwent S-alkylation followed by alpha,alpha-dialkylation with cis-1,4-dichloro-2-butene to form polymer-bound 3-phenylsulfonylcyclopentenes 8. Subsequent epoxidation of the cyclopentene moiety in 8 was accomplished by
Efficient 4+ 1 Syntheses of Highly Functionalized Cyclopentenes.
Nantz MH, et al.
Synthetic Communications, 17(1), 55-69 (1987)
Reactions of cis-and trans-1, 4-dichloro-2-butene with sodium amide.
Heasley VL and Lais BR.
The Journal of Organic Chemistry, 33(6), 2571-2572 (1968)
L S Mullin et al.
Drug and chemical toxicology, 23(3), 403-417 (2000-08-26)
This study was conducted to elucidate the time- and dose-response relationships of long-term, low-level 1,4-dichlorobutene-2 (DCB) inhalation exposure to nasal tumor induction in rats. Male Crl:CD BR rats were exposed 6 hours per day, 5 days week to 0, 0.1
S Phadtare et al.
Nucleic acids symposium series, (18)(18), 25-28 (1987-01-01)
Reaction of adenine (1a) or cytosine (1b) with excess 1,4-dichloro-2-butyne catalyzed by K2CO3 in (CH3)2SO gave the 4-chloro-2-butynyl derivatives 2a and 2b. The latter were converted to the 4-hydroxy-2-butynyl compounds 3a and 3b by refluxing in 0.1 M HCl. Isomerization
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门