所有图片(1)
About This Item
经验公式(希尔记法):
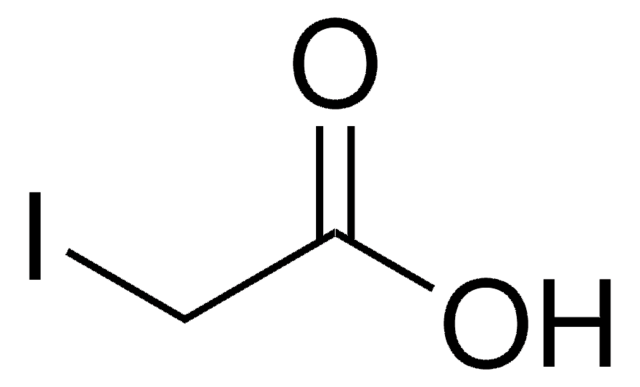
C5H5ClN2O2
CAS号:
分子量:
160.56
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
257 °C (dec.) (lit.)
SMILES 字串
ClCC1=CC(=O)NC(=O)N1
InChI
1S/C5H5ClN2O2/c6-2-3-1-4(9)8-5(10)7-3/h1H,2H2,(H2,7,8,9,10)
InChI 密鑰
VCFXBAPEXBTNEA-UHFFFAOYSA-N
一般說明
6-(Chloromethyl)uracil on chlorination with sulfuryl chloride in acetic acid yields 5-chloro-6-(chloromethyl)uracil.
應用
6-(Chloromethyl)uracil was used in the synthesis of:
- 5-bromo-6-(chloromethyl)uracil
- pteridine compounds, potential anticancer agents
- substituted uracil pyridinium compounds, potential inhibitors of thymidine phosphorylase
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
J Klosa
Arzneimittel-Forschung, 30(2), 228-231 (1980-01-01)
The synthesis of new uracil derivatives is described. In 4-chloromethyluracil, chlorine can be easily exchanged under mild conditions for amine, aniline, hydrazine, and phenol.
Paul E Murray et al.
Bioorganic & medicinal chemistry, 10(3), 525-530 (2002-01-30)
A series of water soluble N(1)- and C(6)-substituted uracil pyridinium compounds were prepared as potential inhibitors of thymidine phosphorylase (TP). The C(6)-uracil substituted derivatives were the most active. 1-[(5-Chloro-2,4-dihydroxypyrimidin-6-yl)methyl]pyridinium chloride, was identified as the best inhibitor being 5-fold more potent
W Wang et al.
International journal of radiation biology, 71(4), 387-399 (1997-04-01)
In this work radicals generated by dissociative electron attachment to iodoacetamide (H2NCOCH2.) and 6-chloromethyluracil (U5CH2.) are suggested to react with DNA nucleotides in frozen aqueous solutions via either hydrogen abstraction or addition to the double bonds of the bases. Methyl
Shingo Yano et al.
Bioorganic & medicinal chemistry, 12(13), 3431-3441 (2004-06-10)
A series of novel 6-methylene-bridged uracil derivatives have been prepared as inhibitors of human thymidine phosphorylase (TP). To enhance the in vivo antitumor activity of fluorinated pyrimidine 2'-deoxyribonucleosides such as 2'-deoxy-5-(trifluoromethyl)uridine (F(3)dThd), a potent TP inhibitor preventing their degradation to
Prem M S Chauhan et al.
Bioorganic & medicinal chemistry, 13(10), 3513-3518 (2005-04-26)
Several pteridine analogues 4-13, 23-26 have been synthesized and tested in vitro against three cancer cell lines, MCF7 (breast), NCI-H460 (lung) and SF-268 (CNS). All tested pteridines can serve as novel templates for the anticancer chemotherapy and can serve as
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门