所有图片(1)
About This Item
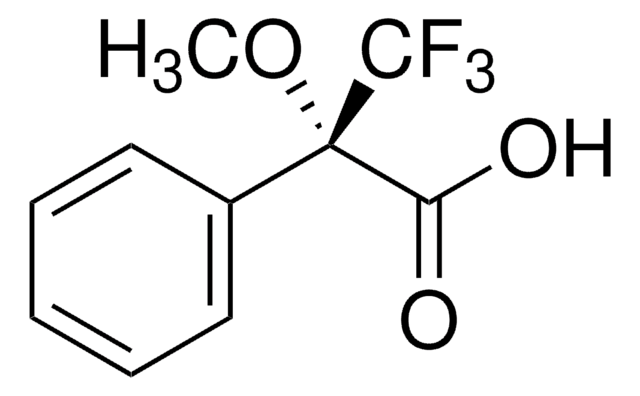
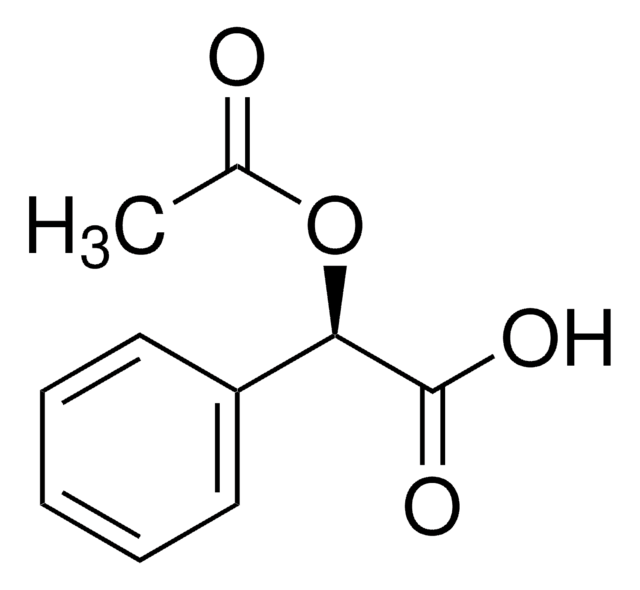
线性分子式:
C6H5C(OCH3)(CF3)CO2H
CAS号:
分子量:
234.17
Beilstein:
3591560
EC號碼:
MDL號碼:
分類程式碼代碼:
12352112
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
99%
形狀
solid
光學活性
[α]20/D +72°, c = 1.6 in methanol
光學純度
ee: 99% (GLC)
折射率
n20/D 1.473 (lit.)
bp
105-107 °C/1 mmHg (lit.)
mp
46-49 °C (lit.)
密度
1.344 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
CO[C@@](C(O)=O)(c1ccccc1)C(F)(F)F
InChI
1S/C10H9F3O3/c1-16-9(8(14)15,10(11,12)13)7-5-3-2-4-6-7/h2-6H,1H3,(H,14,15)/t9-/m1/s1
InChI 密鑰
JJYKJUXBWFATTE-SECBINFHSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(R)-(+)-α-Methoxy-α-trifluoromethylphenylacetic acid is commonly used as a derivatizing agent in Mosher ester analysis, an NMR-based method for determining the absolute configuration of the chiral carbon center in a secondary alcohol.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
其他說明
doi:10.1038/nprot.2007.354
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Tetrahedron Asymmetry, 18, 975-975 (2007)
Lidia De Luca et al.
The Journal of organic chemistry, 72(10), 3955-3957 (2007-04-11)
An effective route to chiral optically active 2-substituted benzofurans directly from carboxylic acids is reported. This procedure, which allows the preparation of alpha-alkyl-2-benzofuranmethanamines from N-protected alpha-amino acids without sensible racemization phenomena, proceeds in good yields under mild conditions with the
Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons.
Hoye TR, et al.
Nature Protocols, 2(10), 2451-2458 (2007)
Giancarlo Cravotto et al.
Chirality, 16(8), 526-533 (2004-08-04)
Cyclodextrin (CD) derivatives are important selectors for analytical chiral recognition. Their enantioselectivities and chemical properties depend on ring size and on nature, number and location of substituents. This paper describes the synthesis of 6-O-TBDMS-2,3-O-methyl beta-cyclodextrins bearing in position 2 either
J Martín Torres-Valencia et al.
Phytochemical analysis : PCA, 13(6), 329-332 (2002-12-24)
A method to determine the absolute configuration of 2,3-epoxy-2-methylbutanoate ester residues in natural products is presented, based on (i) the reduction of the ester function to yield a 2-methyl-1,2-butanediol, (ii) esterification of the obtained primary alcohol with either (R)-(+)- or
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门