推荐产品
化驗
98%
mp
89-91 °C (lit.)
SMILES 字串
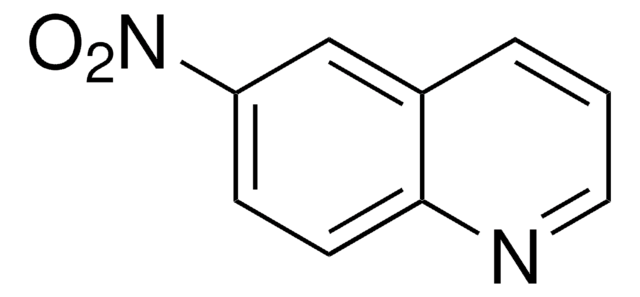
[O-][N+](=O)c1cccc2cccnc12
InChI
1S/C9H6N2O2/c12-11(13)8-5-1-3-7-4-2-6-10-9(7)8/h1-6H
InChI 密鑰
OQHHSGRZCKGLCY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
8-Nitroquinoline was used to prepare furazano [3,4-h] quinoline. It was also used to synthesize corresponding 2-substituted phenoxy-6-methoxy-8-aminoquinoline.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
C Chen et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 27(6), 418-422 (1992-01-01)
2-Substituted phenoxy-, 4-methyl-6-methoxy-8-aminoquinolines and 7-methoxy-5-aminoquinoxaline were condensed with 1-phthalimido-bromo-alkane to yield 2-substituted phenoxy-, 4-methyl-6-methoxy-8-(1-phthalimidoalkyl)-aminoquinolines (compounds 7-10 and 15-20) and 7-methoxy-5-(1-phthalimidoalkyl)aminoquinoxalines (28-30) which were subsequently reacted with hydrazine hydrate to give 2-substituted phenoxy-, 4-methyl-6-methoxy-8-(1-aminoalkyl)-aminoquinolines (11-14 and 22-27) and 7-methoxy-5-(1-aminoalkyl) aminoquinoxalines (31-33)
M Hasegawa et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 121(6), 379-393 (2001-07-04)
A reaction from various kinds of nitroquinoline with hydroxylamine in potassium hydroxide alkalinity produced a novel product, furazanoquinoline, besides the known amino derivatives. The products obtained were furazano [3,4-f] quinoline (5) from 5-nitroquinoline (1) and 6-nitroquinoline (6), and furazano [3,4-h]
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门