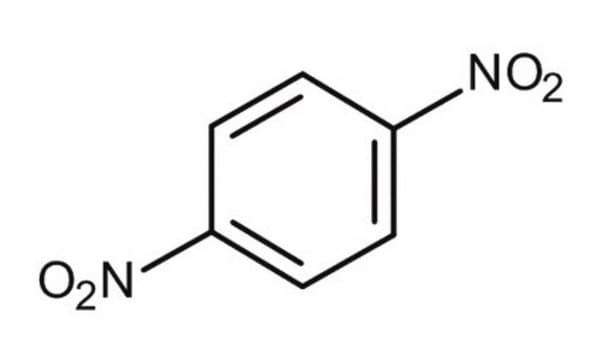
推荐产品
化驗
98%
bp
183.4 °C/34 mmHg (lit.)
mp
170-173 °C (lit.)
溶解度
alcohol: soluble 1g in 300ml
boiling water: soluble 1g in 555ml
cold water: soluble 1g in 12,500ml
benzene: very slightly soluble
chloroform: very slightly soluble
ethyl acetate: very slightly soluble
密度
1.625 g/mL at 25 °C (lit.)
SMILES 字串
[O-][N+](=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H4N2O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H
InChI 密鑰
FYFDQJRXFWGIBS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
準備報告
訊號詞
Danger
危險分類
Acute Tox. 1 Dermal - Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
302.0 °F - closed cup
閃點(°C)
150 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门