B3687
Biliverdin Reductase A human
recombinant, expressed in E. coli, ≥90% (SDS-PAGE), buffered aqueous solution
Synonym(s):
BLVRA, BVRA, Bilirubin: NAD(P)+ oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
recombinant
expressed in E. coli
Quality Level
Assay
≥90% (SDS-PAGE)
form
buffered aqueous solution
specific activity
≥700 units/mg protein
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... BLVRA(644)
General description
Research area: IMMUNO AND CKS. Biliverdinreductase A (BVRA) is an isozyme of the biliverdin reductase. It is a cellsurface membrane receptor. BVRA consists of two major regions, theregulatory/DNA interaction domain and catalytic domain.
Application
Biliverdin Reductase Ahuman has been used as a component of the incubation medium to evaluate theactivity of hemeoxygenase 1. It has also been used as a standard todetermine the age of the bleed from haemorrhagic lesion.
Biochem/physiol Actions
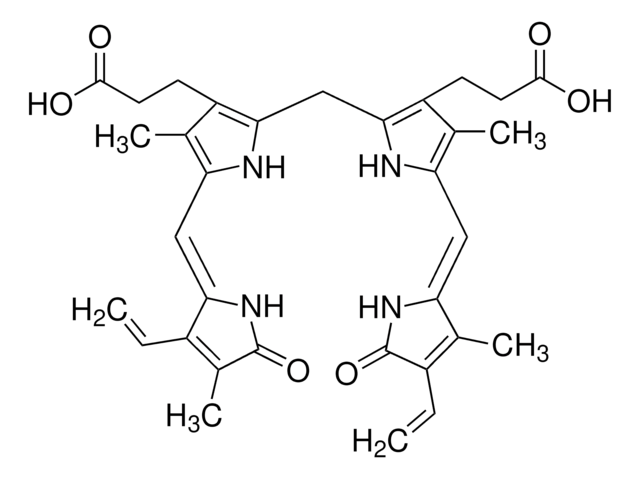
Biliverdin reductase catalyzes the transformation of the blue-green pigment biliverdin IX to the yellow-orange bile pigment bilirubin IX by converting a double-bond between the second and third pyrrole ring into a single-bond. This enzyme has two distinct cofactor-dependent pH optima. In the acidic range of pH 6.0-6.7, NADH is utilized, whereas in the alkaline range of pH 8.5-8.7, NADPH is utilized. Biliverdin reductase is considered a major physiologic cytoprotectant. Biliverdin reductase suppresses experimental autoimmune encephalomyelitis in rats. Depletion of biliverdin reductase leads to accumulation of cellular oxidants and augmented cell death.Biliverdin reductase (BVR)modulates the functions of insulin via the insulin-like growth factor 1 (IGF-1)pathway. It shows dual specificity kinases where it phosphorylates tyrosineapart from serine and threonine. BVR acts as a biomarker in certain cancers.
Unit Definition
1 unit of biliverdin reductase will transform 1 nanomole of biliverdin to bilirubin per minute in an NADPH dependent reaction at pH 8.5 using 100 mM K-phosphate buffer at 37 °C.
Physical form
Solution containing 20 mM potassium phosphate buffer, 10% glycerol, 0.2% Igepal CA630, 1 mM EDTA, and 0.1 mM DTT.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Luke O'Brien et al.
Trends in endocrinology and metabolism: TEM, 26(4), 212-220 (2015-03-03)
The biliverdin reductase (BVR) isozymes BVRA and BVRB are cell surface membrane receptors with pleiotropic functions. This review compares, for the first time, the structural and functional differences between the isozymes. They reduce biliverdin, a byproduct of heme catabolism, to
Ali Abbasi et al.
Nutrition and cancer, 73(10), 2003-2013 (2020-09-15)
To assess the effect of sequential treatment with Vitamin C (VC) and Quercetin (Q) on Nrf2-related oxidative stress in PC3 and DU145 cells, viability was measured by MTT assay. Intracellular ROS levels were determined, using 2'-7'-dichlorodihydrofluorescein diacetate fluorescent as a
Rayyan Manwar et al.
Journal of biophotonics, 16(7), e202200316-e202200316 (2023-03-31)
The onset of intracerebral hemorrhage and its progression toward acute brain injury have been correlated with the concentration of unconjugated bilirubin (BR). In addition, BR has been considered a novel predictor of outcome from intracranial hemorrhage. Since the existing invasive
Peter E M Gibbs et al.
Frontiers in pharmacology, 6, 119-119 (2015-06-20)
Biliverdin reductase (BVR) is a multifunctional protein that is the primary source of the potent antioxidant, bilirubin. BVR regulates activities/functions in the insulin/IGF-1/IRK/PI3K/MAPK pathways. Activation of certain kinases in these pathways is/are hallmark(s) of cancerous cells. The protein is a
W Uerkvitz et al.
The Journal of biological chemistry, 256(1), 382-389 (1981-01-10)
Three periplasmic nucleoside monophosphate-splitting phosphatases from Salmonella typhimurium, 2':3'-cyclic nucleotide 2'-phosphodiesterase, nonspecific acid phosphatase I, and nonspecific acid phosphatase II, were separated by column chromatography. They are characterized with respect to their substrate specificities, Km values, pH optima, molecular weights
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service