C2174005
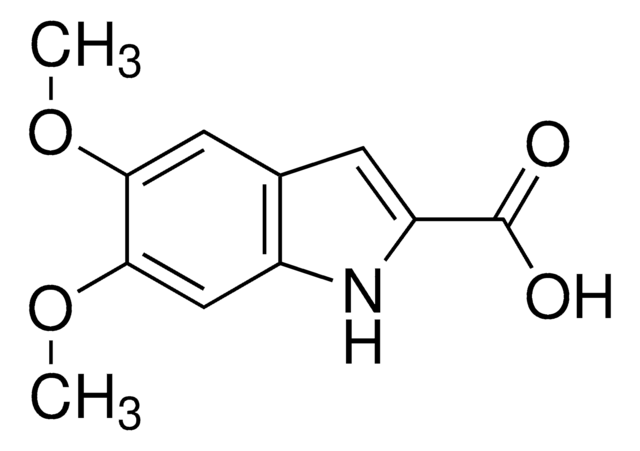
Cilazapril impurity D
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
(1S,9S)-9-{[(R)-1-Ethoxycarbonyl-3-phenylpropyl]amino}-10- oxo-octahydro-6H-pyridazino[1,2a][1,2]diazepine-1-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H31N3O5
CAS Number:
Molecular Weight:
417.50
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
cilazapril
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Cilazapril impurity D EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Leszek Tylicki et al.
Scandinavian journal of urology and nephrology, 42(4), 381-388 (2009-02-21)
Despite the proven effectiveness of combination therapy with an angiotensin I-converting enzyme inhibitor (ACEI) and angiotensin II-receptor blockers (ARBs) for the prevention and treatment of kidney disease, it has not proved possible to inhibit the progress of chronic nephropathies completely.
IgA nephropathy: a disease in search of a large-scale clinical trial to reliably inform practice.
Giovanni F M Strippoli et al.
American journal of kidney diseases : the official journal of the National Kidney Foundation, 53(1), 5-8 (2008-12-23)
Mustafa Tuncer et al.
Advances in therapy, 25(2), 99-105 (2008-02-26)
P-wave dispersion (PWD) has been shown to be a non-invasive electrocardiographic predictor for development of atrial fibrillation (AF). Thus, it may be possible to decrease AF risk through improvement in PWD. Our objective was to compare the effects of cilazapril
High-dose angiotensin-converting enzyme inhibitor attenuates oxidative stress in patients with chronic kidney disease.
Marcin Renke et al.
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 24(2), 689-690 (2008-12-05)
Eduardo Cantoni Rosa et al.
Arquivos brasileiros de endocrinologia e metabologia, 52(1), 65-75 (2008-03-18)
Blood pressure (BP) and target organ responses to antihypertensive drugs are not well established in hypertensive obese patients. This study is aimed at evaluating the effects of obesity and adiposity distribution patterns on these responses. 49 hypertensive obese women were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service