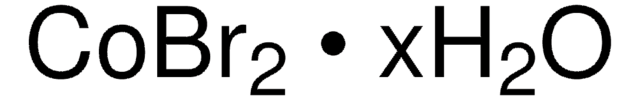03858
Dimethylglyoxime
≥97.0% (TLC)
Synonym(s):
dmgH2, 2,3-Butanedione dioxime, Diacetyldioxime
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(=NOH)C(=NOH)CH3
CAS Number:
Molecular Weight:
116.12
Beilstein:
506731
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (TLC)
form
powder
mp
240-241 °C (lit.)
SMILES string
CC(=N/O)\C(C)=N\O
InChI
1S/C4H8N2O2/c1-3(5-7)4(2)6-8/h7-8H,1-2H3/b5-3+,6-4+
InChI key
JGUQDUKBUKFFRO-GGWOSOGESA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dimethylglyoxime can be used:
- As a ligand for the synthesis of cobaloxime complexes which are used as electrocatalysts for the production of hydrogen by electroreduction of protons.
- As a precipitant for the synthesis of NiO and CuI nanoparticles.P
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods.
Li X, et al.
Solid State Communications, 137(11), 581-584 (2006)
Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases.
Razavet M, et al.
Inorganic Chemistry, 44(13), 4786-4795 (2005)
Jean-Marie Lachapelle et al.
Dermatology (Basel, Switzerland), 209(4), 288-290 (2004-11-13)
To determine the release of nickel from 1- and 2-euro coins and the ability to produce allergic contact dermatitis from the application of coins to the palmar skin of nickel-sensitized individuals. Three experiments were conducted. Experiments 1 and 2 checked
Jacob Pontoppidan Thyssen et al.
Journal of the American Academy of Dermatology, 58(6), 1000-1005 (2008-04-15)
Nickel sensitization is frequent among US patients with dermatitis and in the general population. In Europe, decreasing prevalences of nickel sensitization are observed as a result of the European Union Nickel Directive. However, no directive exists in the United States.
Carsten R Hamann et al.
Contact dermatitis, 62(4), 232-240 (2010-03-20)
China and Thailand produce large amounts of jewellery that are sold domestically and abroad. To identify nickel release and metal content in earrings purchased in China and Thailand. A total of 557 earrings were randomly purchased from vendors in 11
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service