All Photos(1)
About This Item
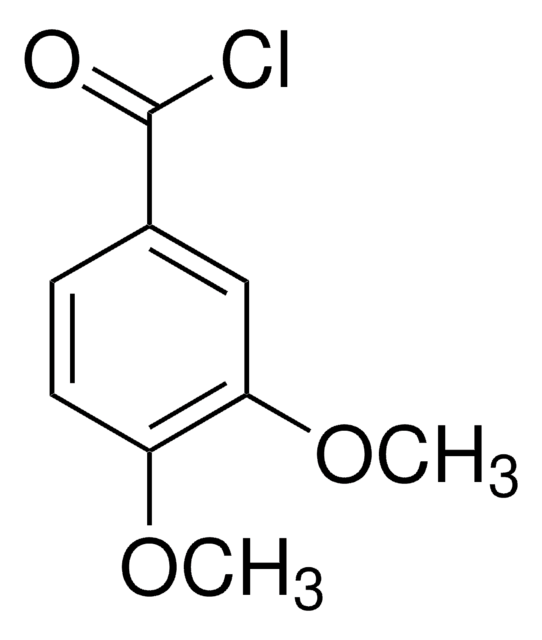
Linear Formula:
(CH3O)2C6H3CO2H
CAS Number:
Molecular Weight:
182.17
Beilstein:
511834
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
178-180 °C (lit.)
SMILES string
COc1cc(OC)cc(c1)C(O)=O
InChI
1S/C9H10O4/c1-12-7-3-6(9(10)11)4-8(5-7)13-2/h3-5H,1-2H3,(H,10,11)
InChI key
IWPZKOJSYQZABD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Dimethoxybenzoic acid (3,5-DmeoxBA) can be used as a reactant for the synthesis of:
It can also be used as a ligand to synthesize lanthanide complexes [Ln(3,5-DmeoxBA)3(phen)]2; where phen is 1,10-phenanthroline.
- 5,7-Dimethoxy-3,4-diphenylisocoumarin by coupling with diphenylacetylene.
- Biotin dimedone, a reagent used in the study of protein sulfenation.
It can also be used as a ligand to synthesize lanthanide complexes [Ln(3,5-DmeoxBA)3(phen)]2; where phen is 1,10-phenanthroline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Crystal structures, luminescence, and thermodynamic properties of lanthanide complexes with 3, 5-dimethoxybenzoic acid and 1, 10-phenanthroline.
Zheng JR, et al.
The Journal of Chemical Thermodynamics, 57(9), 169-177 (2013)
Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue.
Charles RL, et al.
Molecular and Cellular Proteomics, 6(9), 1473-1484 (2007)
Rhodium-and iridium-catalyzed oxidative coupling of benzoic acids with alkynes via regioselective C- H bond cleavage.
Ueura K, et al.
The Journal of Organic Chemistry, 72(14), 5362-5367 (2007)
Marta Olech et al.
Molecules (Basel, Switzerland), 25(8) (2020-04-25)
Fast and reliable determination of polyphenols is a quite common goal during investigation of new plant materials and herbal products, their standardization, quality control, or chemo-taxonomical studies. The aim of this study was to develop and validate methods based on
Alla V Lipeeva et al.
European journal of medicinal chemistry, 100, 119-128 (2015-06-17)
A series of 2-(4-R-triazolyl)substituted 3-oxo-2,3-dihydrofurocoumarins have been synthesized by a regioselective cycloaddition of 2-azidooreoselone 1 or 2-azido-9-[(4-methylpiperazin-1-yl)methyl]oreoselone 2 with various alkynes in the presence of Cu(II)/ascorbate in water/methylene chloride reaction medium. The structure of 2-azidooreoselone was established by X-ray structure
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service