All Photos(1)
About This Item
Empirical Formula (Hill Notation):
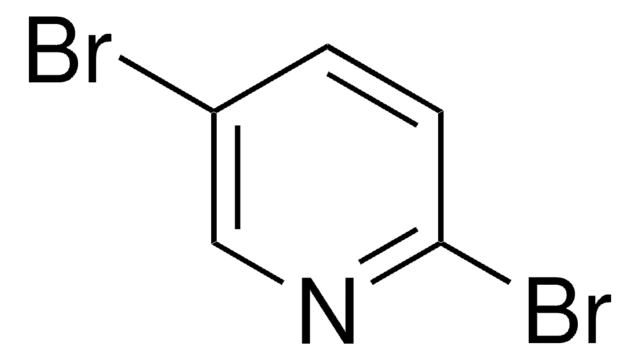
C5H3BrN2O2
CAS Number:
Molecular Weight:
202.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
148-150 °C (lit.)
SMILES string
[O-][N+](=O)c1ccc(Br)cn1
InChI
1S/C5H3BrN2O2/c6-4-1-2-5(7-3-4)8(9)10/h1-3H
InChI key
ATXXLNCPVSUCNK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure-based design and synthesis of the first weak non-phosphate inhibitors for IspF, an enzyme in the non-mevalonate pathway of isoprenoid biosynthesis.
Baumgartner C, et al.
Helvetica Chimica Acta, 90(6), 1043-1068 (2007)
FT-Raman and FT-IR spectra, vibrational assignments and density functional studies of 5-bromo-2-nitropyridine.
Sundaraganesan N, et al.
Spectrochimica Acta Part A: Molecular Spectroscopy, 61(13), 2995-3001 (2005)
Synthesis of fluorine-18-labelled 5-and 6-fluoro-2-pyridinamine.
Abrahim A, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 49(4), 345-356 (2006)
Lixin Qiao et al.
Bioorganic & medicinal chemistry letters, 19(21), 6122-6126 (2009-09-29)
A structure-activity relationship study for a 2-chloroanilide derivative of pyrazolo[1,5-a]pyridine revealed that increased EphB3 kinase inhibitory activity could be accomplished by retaining the 2-chloroanilide and introducing a phenyl or small electron donating substituents to the 5-position of the pyrazolo[1,5-a]pyridine. In
Susheel J Nara et al.
The Journal of organic chemistry, 73(23), 9326-9333 (2008-11-04)
A convenient approach to 3-pyridinols and 5-pyrimidinols via a two-step Cu-catalyzed benzyloxylation/catalytic hydrogenation sequence is presented. The corresponding 3-pyridinamines and 5-pyrimidinamines can be prepared in an analogous sequence utilizing benzylamine in lieu of benzyl alcohol. The radical-scavenging ability of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)