All Photos(1)
About This Item
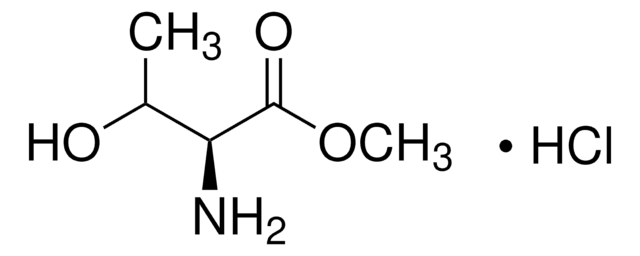
Linear Formula:
HOCH2CH(NH2)CO2CH3·HCl
CAS Number:
Molecular Weight:
155.58
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
optical activity
[α]20/D −4°, c = 4 in ethanol
reaction suitability
reaction type: solution phase peptide synthesis
mp
163-166 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.COC(=O)[C@H](N)CO
InChI
1S/C4H9NO3.ClH/c1-8-4(7)3(5)2-6;/h3,6H,2,5H2,1H3;1H/t3-;/m1./s1
InChI key
NDBQJIBNNUJNHA-AENDTGMFSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carlos Aydillo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(17), 4840-4848 (2007-03-17)
A new chiral serine equivalent and its enantiomer have been synthesized from (S)- and (R)-N-Boc-serine methyl esters (Boc: tert-butyloxycarbonyl). The use of these compounds as chiral building blocks has been demonstrated in the synthesis of alpha-alkyl alpha-amino acids by diastereoselective
Cédric Couturier et al.
Organic letters, 8(10), 2183-2186 (2006-05-05)
[reaction: see text] Reaction of N,N-dibenzyl-O-methylsulfonyl serine methyl ester with a variety of heteronucleophiles (sodium azide, sodium phthalimide, amines, thiols) and carbanions (sodium malonate) gave, via an aziridinium intermediate, the corresponding beta-amino or alpha,beta-diamino ester in good to excellent yield.
J Sélambarom et al.
Carbohydrate research, 330(1), 43-51 (2001-02-24)
The reaction of L-serine methyl ester hydrochloride (1) with paraformaldehyde (2) in dichloromethane in the presence of triethylamine afforded a novel compound: [lS,2S,6S,7S]-1,6-diaza-4,9-dioxa-2,7-dimethoxycarbonylbicyclo[4.4.1]undecane (4) as a 2:3 adduct of 1 with 2. 1H and 13C NMR spectroscopy were unable to
Dragana Ahel et al.
FEBS letters, 579(20), 4344-4348 (2005-08-02)
Seryl-tRNA synthetases (SerRSs) fall into two distinct evolutionary groups of enzymes, bacterial and methanogenic. These two types of SerRSs display only minimal sequence similarity, primarily within the class II conserved motifs, and possess distinct modes of tRNA(Ser) recognition. In order
Yu Harayama et al.
Chemical communications (Cambridge, England), (13)(13), 1764-1766 (2005-03-26)
The use of hypervalent iodine(III) reagents allowed us to develop the novel and efficient direct synthesis of N,O-acetal compounds via the oxidative fragmentation reaction of alpha-amino acids or alpha-amino alcohols; furthermore, we succeeded in developing an improved synthesis of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service