All Photos(1)
About This Item
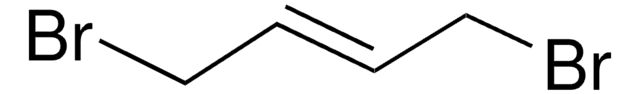
Linear Formula:
ClCH2CH=CHCH2Cl
CAS Number:
Molecular Weight:
125.00
Beilstein:
1719693
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
10 mmHg ( 20 °C)
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.488 (lit.)
bp
74-76 °C/40 mmHg (lit.)
mp
1-3 °C (lit.)
density
1.183 g/mL at 25 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
ClC\C=C\CCl
InChI
1S/C4H6Cl2/c5-3-1-2-4-6/h1-2H,3-4H2/b2-1+
InChI key
FQDIANVAWVHZIR-OWOJBTEDSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans-1,4-Dichloro-2-butene may be used for the alkylation of adenine to 9-alkylpurines. It can react with diethyl malonate to form vinylcyclopropane derivative. It can also undergo asymmetric allylic alkylation with Grignard reagents in the presence of copper thiophene carboxylate catalyst.
Other Notes
85%, remainder predominantly cis isomer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
127.4 °F - closed cup
Flash Point(C)
53 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High Diversity on Simple Substrates: 1, 4?Dihalo?2?butenes and Other Difunctionalized Allylic Halides for Copper?Catalyzed SN2? Reactions.
Falciola C A, et al.
Chemistry?A European Journal , 14(34), 10615-10627 (2008)
Improved selectivity in the preparation of some 1, 1-difunctionalized 3-cyclopentenes. High yield synthesis of 3-cyclopentenecarboxylic acid.
Depres JP and Greene AE.
The Journal of Organic Chemistry, 49(5), 928-931 (1984)
S Phadtare et al.
Journal of medicinal chemistry, 30(2), 437-440 (1987-02-01)
Alkylation of adenine (5a) or 2-amino-6-chloropurine (5b) with excess trans-1,4-dichloro-2-butene (4), effected by K2CO3 in dimethyl sulfoxide or tetra-n-butylammonium fluoride in tetrahydrofuran, led in 90-95% regioselectivity to 9-alkylpurines 6a and 6b. The title compounds 2a and 2b were obtained by
R J Gardner et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 23(1), 87-92 (1985-01-01)
Several important chemicals, including formaldehyde, 1,4-dichloro-2-butene, bis-chloromethyl ether, hexamethylphosphoramide, and epichlorohydrin have been shown to produce nasal tumours in rats following repeated or continuous inhalation exposures. Some of these compounds are respiratory irritants. To determine whether there is a correlation
S Phadtare et al.
Nucleic acids symposium series, (18)(18), 25-28 (1987-01-01)
Reaction of adenine (1a) or cytosine (1b) with excess 1,4-dichloro-2-butyne catalyzed by K2CO3 in (CH3)2SO gave the 4-chloro-2-butynyl derivatives 2a and 2b. The latter were converted to the 4-hydroxy-2-butynyl compounds 3a and 3b by refluxing in 0.1 M HCl. Isomerization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service