All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O
CAS Number:
Molecular Weight:
120.15
Beilstein:
111928
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.549 (lit.)
bp
188-189 °C (lit.)
solubility
alcohol: soluble
carbon disulfide: soluble
chloroform: soluble
diethyl ether: soluble
density
1.065 g/mL at 25 °C (lit.)
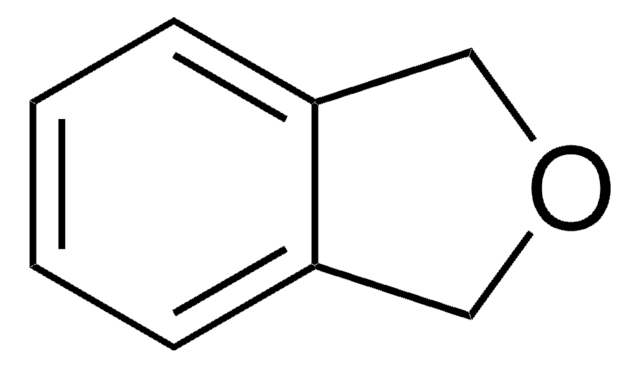
SMILES string
C1Cc2ccccc2O1
InChI
1S/C8H8O/c1-2-4-8-7(3-1)5-6-9-8/h1-4H,5-6H2
InChI key
HBEDSQVIWPRPAY-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
Biotransformation of 2,3-dihydrobenzofuran using intact cells of Pseudomonas putida UV4 has been investigated. 2,3-Dihydrobenzofuran is the intermediate formed during catalytic hydrodeoxygenation of benzofuran.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Leticia Jiménez-González et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(34), 8762-8769 (2006-09-06)
2,3-Dihydrobenzofurans can be diastereoselectively prepared by condensation of aromatic aldehydes with 2,3-dihydrobenzoxasilepines under the catalysis of Ag(I) complexes, and in the presence of a source of fluoride ion. The application of this strategy by using chiral catalysts leads to a
H S Heine et al.
Chemico-biological interactions, 59(2), 219-230 (1986-09-01)
The effects of dietary administration of equimolar doses (5 mmol/kg body wt per day) of trimethylene oxide, trimethylene sulfide, coumaran, benzofuran, indole, and indole-3-carbinol on the activities of microsomal epoxide hydrolase and several other xenobiotic metabolizing enzymes were measured in
S Antus et al.
Chirality, 13(8), 493-506 (2001-07-24)
The correlation between the helicity (absolute conformation) of the O-heterocyclic ring of chiral 2,3-dihydrobenzo[b]furan (1) and chromane (2) derivatives and their (1)L(b) band CD was investigated. The same helicity rule was found for both unsubstituted chromophores: P/M helicity of the
S Ohkawa et al.
Journal of medicinal chemistry, 40(4), 559-573 (1997-02-14)
A series of 2,3-dihydro-5-benzofuranamines (5-aminocoumarans) were developed for the treatment of traumatic and ischemic central nervous system (CNS) injury. Compounds within this class were extremely effective inhibitors of lipid peroxidation in vitro and antagonized excitatory behavior coupled with peroxidative injury
Prashant P Deshpande et al.
Journal of industrial microbiology & biotechnology, 35(8), 901-906 (2008-05-23)
Microbial hydroxylation of o-bromophenylacetic acid provided 2-bromo-5-hydroxyphenylacetic acid. This enabled a route to the key intermediate 4-bromo-2,3-dihydrobenzofuran for synthesizing a melatonin receptor agonist and sodium hydrogen exchange compounds. Pd-mediated coupling reactions of 4-bromo-2,3-dihydrobenzofuran provided easy access to the 4-substituted-2,3-dihydrobenzofurans.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service