All Photos(1)
About This Item
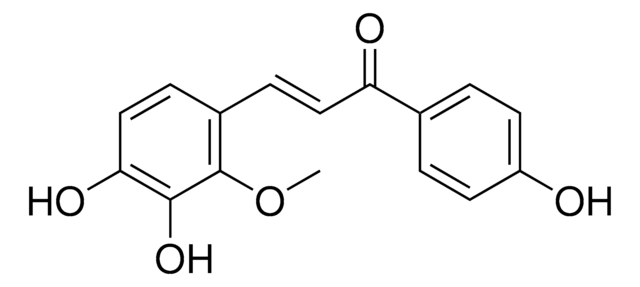
Empirical Formula (Hill Notation):
C59H83N17O14
Molecular Weight:
1254.40
UNSPSC Code:
12352209
NACRES:
NA.32
Recommended Products
Assay
≥95% (HPLC)
form
lyophilized
composition
Peptide Content, ≥72%
storage condition
protect from light
storage temp.
−20°C
Amino Acid Sequence
Glp-His-Trp-Ser-Tyr-Lys-Leu-Arg-Pro-Gly
Application
Luteinizing-hormone-releasing hormone (LHRH) (GnRH) is a decapeptide (pyroQHWSYKLRPG-NH2) trophic hormone that regulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. Various analogues have been developed using D-amino acids to increase resistance to degradation. These analogues are used as potential targeting agents in the development of chemotherapies and as possible agonists and antagonist of the LHRH receptor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Andreas R Günthert et al.
Breast cancer research and treatment, 87(3), 255-264 (2004-11-06)
More than 50% of human breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH-R). These receptors can be used for targeted chemotherapy with agents like AN-152, in which doxorubicin is linked to analog [D-Lys6]LHRH. We compared the effects of AN-152
Alexandra P Kafka et al.
Biomedical chromatography : BMC, 24(2), 132-139 (2009-06-12)
A high-performance liquid chromatography (HPLC) method for assay of d-Lys(6)-GnRH contained in a microemulsion-type formulation is described. The peptide is extracted from the microemulsion matrix and quantified using a two-step gradient method. Separation from microemulsion compounds and potential peptide oxidation
G Emons et al.
European journal of cancer & clinical oncology, 25(2), 215-221 (1989-02-01)
As a first step to investigate whether gonadotropin releasing hormone (GnRH) analogs might be able to modulate directly the proliferation of human epithelial ovarian carcinomata, we checked if binding sites for GnRH are present in these malignancies. Specific binding of
Ana M Bajo et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 9(10 Pt 1), 3742-3748 (2003-09-25)
The receptors for luteinizing hormone-releasing hormone receptor (LHRH-R) are found in >50% of human breast cancers. Doxorubicin (DOX) was linked to [D-Lys(6)]LHRH to form a cytotoxic conjugate, AN-152, which can be targeted to tumor cells expressing LHRH-R. We evaluated the
P J Wormald et al.
The Journal of clinical endocrinology and metabolism, 61(6), 1190-1194 (1985-12-01)
A specific, high affinity receptor for GnRH in human pituitaries obtained post mortem is described. The human pituitary GnRH receptor bound GnRH, a GnRH agonist [(D-Ala6,N alpha-MeLeu7,Pro9NEt)-GnRH], and a GnRH antagonist [Ac-D-Nal(2)1,D-alpha-Me-4-ClPhe2,D-3-Pal3,D-Arg6,D-Ala10 )-GnRH] with similar affinities (KdS of 4.81 nM
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[D-Trp6]-LH-RH ≥97% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/321/602/34cc2814-2f6f-4fc9-a266-835bfe27bcc5/640/34cc2814-2f6f-4fc9-a266-835bfe27bcc5.png)