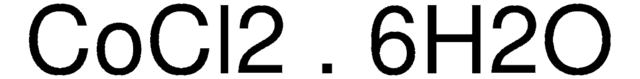8.02540
Cobalt(II) chloride anhydrous
for synthesis
Synonym(s):
Cobalt(II) chloride anhydrous, Cobalt dichloride
About This Item
Recommended Products
grade
anhydrous
Quality Level
form
powder
potency
418 mg/kg LD50, oral (Rat)
mp
735 °C
solubility
soluble 585.9 g/L
density
3.36 g/cm3 at 25 °C
storage temp.
2-30°C
InChI
1S/2ClH.Co/h2*1H;/q;;+2/p-2
InChI key
GVPFVAHMJGGAJG-UHFFFAOYSA-L
Application
CoCl2 can be used as a catalyst:
- For the dealkylation of tertiary amines.
- In the synthesis of thioketones and thioaldehydes via thionation of carbonyl compounds in the presence of bis(trimethylsilyl) sulfide.
- In the oxidation of aromatic and aliphatic aldehydes to carboxylic acids in the presence of molecular oxygen and acetic anhydride.
- In the stereoselective opening of epoxides with N-substituted anilines.
CoCl2 can also be used as:
- A precursor to synthesize magnetic cobalt nanorods via decomposition of the cobalt coordination complex.
- A catalyst to prepare acid anhydrides by the reaction of acid chlorides with carboxylic acids.
- A reagent in the carbon-carbon bond-forming reactions involving active methylene compounds.
Analysis Note
Water (K. F.): ≤ 2.0 %
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service