481407
NF-κB Activation Inhibitor
InSolution, ≥98%
Synonym(s):
InSolution NF-κB Activation Inhibitor
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
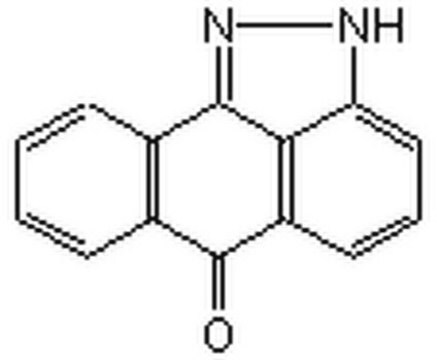
C22H20N4O
Molecular Weight:
356.42
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
shipped in
wet ice
storage temp.
2-8°C
General description
A cell-permeable quinazoline compound that acts as a potent inhibitor of NF-κB transcriptional activation (IC50 = 11 nM in Jurkat cells) and LPS-induced TNF-α production (IC50 = 7 nM in murine splenocytes). Does not exhibit cellular cytotoxicity even at concentrations as high as 10 µM. Reported to exhibit anti-inflammatory effects on carregeenin-induced paw edema in rats.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
NF-κB transcriptional activation
NF-κB transcriptional activation
Product does not compete with ATP.
Reversible: no
Target IC50: 11 nM against NF-κB transcriptional activation in Jurkat cells; 7 nM against LPS-induced TNF-α production in murine splenocytes
Packaging
Packaged under inert gas
Warning
Toxicity: Toxic (F)
Physical form
A 10 mM (1 mg/281 µl) solution of NF-κB Activation Inhibitor (Cat. No. 481406) in DMSO.
Reconstitution
Following initial use, aliquot and refrigerate (4°C).
Other Notes
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Tobe, M., et al. 2003. Bioorg. Med. Chem.11, 383.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
188.6 °F - closed cup - (Dimethylsulfoxide)
Flash Point(C)
87 °C - closed cup - (Dimethylsulfoxide)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation.
Masanori Tobe et al.
Bioorganic & medicinal chemistry, 11(3), 383-391 (2003-01-09)
We disclose here a new structural class of low-molecular-weight inhibitors of NF-kappa B activation that were designed and synthesized by starting from quinazoline derivative 6a. Structure-activity relationship (SAR) studies based on 6a elucidated the structural requirements essential for the inhibitory
Fatmah A Mansour et al.
Oncoimmunology, 9(1), 1729299-1729299 (2020-04-22)
The T-cell inhibitory molecule PD-L1 is expressed on a fraction of breast cancer cells. The distribution of PD-L1 on the different subpopulations of breast cancer cells is not well-defined. Our aim was to study the expression level of PD-L1 on
Differential regulation of transcription factor T-bet induction during NK cell development and T helper-1 cell differentiation.
Fang, et al.
Immunity, 55, 639-655 (2023)
Eunbi Ko et al.
Experimental dermatology, 24(12), 936-941 (2015-07-16)
House dust mites (HDMs) are known to trigger chronic inflammation through Toll-like receptors (TLRs) and their signalling cascades. In this study, we found that TLR2 ligation by HDMs induced the activation of dual oxidase 2 (Duox2) and nuclear factor-κB (NF-κB)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service