T-911
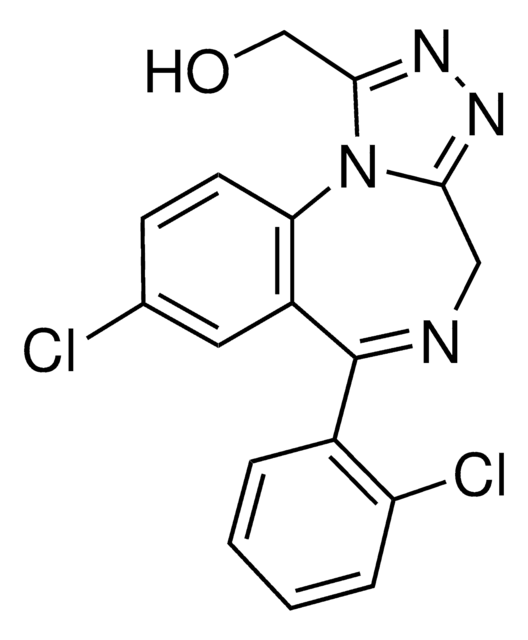
α-Hydroxytriazolam solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
2-8°C
SMILES string
OCc1nnc2CN=C(c3ccccc3Cl)c4cc(Cl)ccc4-n12
InChI
1S/C17H12Cl2N4O/c18-10-5-6-14-12(7-10)17(11-3-1-2-4-13(11)19)20-8-15-21-22-16(9-24)23(14)15/h1-7,24H,8-9H2
InChI key
BHUYWUDMVCLHND-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Pharmacokinetic analysis of benzodiazepines: alpha-Hydroxytriazolam solution is employed in the quantitation of benzodiazepines and their metabolites in biological fluids using liquid chromatography-tandem mass spectrometry, providing accurate pharmacokinetic profiles that are essential for clinical and forensic analysis (Zheng et al., 2018).
- Receptor binding studies: The application of high-purity alpha-Hydroxytriazolam in receptor binding studies enables researchers to elucidate the interaction mechanisms of benzodiazepine analogs at the molecular level, thereby enhancing the understanding of their pharmacological effects (Minegishi et al., 2021).
- Development of analytical methods: alpha-Hydroxytriazolam solution serves as a critical analytical standard in the development of robust assays for the detection and quantification of benzodiazepines in various matrices, facilitating advancements in toxicological and pharmacological research (Lahr et al., 2020).
- Forensic and clinical toxicology: Utilized in forensic science, alpha-Hydroxytriazolam solution aids in the rapid determination of benzodiazepines and their metabolites in urine samples, crucial for the accurate interpretation of toxicological data (Jeong et al., 2015).
- Scientific research in biochemistry: alpha-Hydroxytriazolam research compounds are vital for studying the metabolic pathways and degradation profiles of triazolam and related benzodiazepines, supporting biochemists in drug metabolism and pharmacokinetics research (Jin et al., 2011).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service