P25485
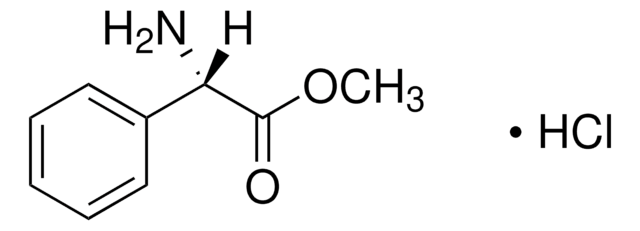
D−(−)-α-Phenylglycine
99%, detection
Synonym(s):
(R)-(−)-2-Phenylglycine, D-2-Phenylglycine, R-(−)-α-Aminophenylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
151.16
Beilstein:
2208676
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
D−(−)-α-Phenylglycine, 99%
Quality Level
Assay
99%
form
powder or crystals
optical activity
[α]20/D −155°, c = 1 in 1 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
302 °C (dec.) (lit.)
application(s)
detection
SMILES string
N[C@@H](C(O)=O)c1ccccc1
InChI
1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m1/s1
InChI key
ZGUNAGUHMKGQNY-SSDOTTSWSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
José Alixandre de Sousa Luis et al.
Molecules (Basel, Switzerland), 15(1), 128-137 (2010-01-30)
Hydantoins and their derivatives constitute a group of pharmaceutical compounds with anticonvulsant and antiarrhythmic properties, and are also used against diabetes. N-3 and C-5 substituted imidazolidines are examples of such products. As such, we have developed a synthesis of 2,4-dione
Shohei Tashiro et al.
Inorganic chemistry, 50(1), 4-6 (2010-12-01)
The optically active cobalt(III) complex with chiral cyclen, (2S,5S,8S,11S)-2,5,8,11-tetraethyl-1,4,7,10-tetraazacyclododecane, preferentially binds to D-phenylglycine (D-Phg) or D-t-leucine (D-t-Leu) rather than L-Phg or L-t-Leu, respectively, with 20% de in dimethyl sulfoxide at 293 K. Comparative studies on the crystal structures of cobalt(III)
Martijn A Hoeben et al.
Biotechnology and bioengineering, 93(4), 607-617 (2006-01-06)
The interfacial partitioning behavior of ampicillin and phenylglycine crystals in different two-phase systems has been investigated. The two-phase systems employed are water/dodecane, water/1-butanol, and water/pentane/methanol. By means of partition experiments and microscopic imaging, it has been shown that the mechanism
Hyung Min Kim et al.
The Journal of chemical physics, 128(18), 184313-184313 (2008-06-06)
We investigated the conformational structures of L-phenylglycine in the gas phase by photoionization and double resonance spectroscopy techniques as well as high-level ab initio calculations. The UV-UV and IR-UV double resonance spectroscopy suggested that there exists only one conformer that
Caroline Haurena et al.
The Journal of organic chemistry, 75(8), 2645-2650 (2010-03-23)
A range of alpha-amino esters has been synthesized in good to high yields using a straightforward three-component reaction among preformed or in situ generated aromatic or benzylic organozinc reagents, primary or secondary amines, and ethyl glyoxylate. The procedure, which is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service