All Photos(1)
About This Item
Linear Formula:
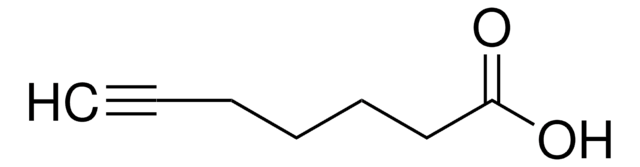
HC≡C(CH2)8CO2H
CAS Number:
Molecular Weight:
182.26
Beilstein:
1704918
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
bp
180 °C/15 mmHg (lit.)
mp
40-42 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)CCCCCCCCC#C
InChI
1S/C11H18O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h1H,3-10H2,(H,12,13)
InChI key
OAOUTNMJEFWJPO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
10-Undecynoic acid (10- UDYA, UDY) is an acetylenic fatty acid. It is reported as highly selective irreversible inhibitor of hepatic ω- and ω-1-lauric acid hydroxylases. Enzyme catalyzed esterification of 10-undecynoic acid has been reported. UDY has been reported to be synthesized by the dehydrobromination of 10-undecenoic acid.
Application
10-Undecynoic acid was employed as model compound to investigate the microwave assisted surface click reactions catalyzed with Cu(II)/sodium L-ascorbate.†
It may be used:
It may be used:
- As a biochemical probe in an assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection.
- To form molecular layers by adsorbing on the fluorite surface.
- In the supercritical hydrothermal synthesis of iron oxide nanoparticles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R C Zangar et al.
Archives of biochemistry and biophysics, 337(2), 217-224 (1997-01-15)
CYP2B, CYP4A, and CYP2E1 mRNA levels are elevated in response to pathophysiological conditions, such as diabetes, high-fat diet, and fasting, in which lipids and ketone bodies are increased. In order to avoid confounding hormonal effects, we utilized primary rat hepatocytes
Formation and wetting characteristics of adsorbed layers of unsaturated carboxylic acids at a fluorite surface.
Drelich J, et al.
Journal of Colloid and Interface Science, 178(2), 720-732 (1996)
P R Ortiz de Montellano et al.
The Journal of biological chemistry, 259(7), 4136-4141 (1984-04-10)
The terminal acetylenic analogue of lauric acid, 11-dodecynoic acid (11-DDYA), specifically inactivates hepatic cytochrome P-450 enzymes that catalyze omega- and omega-1-hydroxylation of lauric acid. The inactivation, as required for a suicidal process, is NADPH- and time-dependent and follows pseudo-first order
M C Romano et al.
Analytical biochemistry, 170(1), 83-93 (1988-04-01)
An assay for the microsomal hydroxylation of lauric acid (LA), based on HPLC with flow-through radiochemical detection, has been developed. Conditions were optimized for resolution and quantitation of three microsomal metabolites of LA, one of which has not been reported
Studies of lipase-catalyzed esterification reactions of some acetylenic fatty acids.
Jie MSFLK and Xun F.
Lipids, 33(1), 71-75 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service