All Photos(3)
About This Item
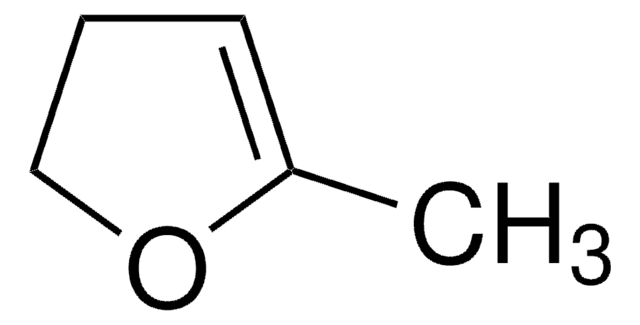
Empirical Formula (Hill Notation):
C4H6O
CAS Number:
Molecular Weight:
70.09
Beilstein:
103168
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
14.46 psi ( 55 °C)
3.67 psi ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
54-55 °C (lit.)
density
0.927 g/mL at 25 °C (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
C1CC=CO1
InChI
1S/C4H6O/c1-2-4-5-3-1/h1,3H,2,4H2
InChI key
JKTCBAGSMQIFNL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides was studied.
Application
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-11.2 °F - closed cup
Flash Point(C)
-24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claudia G Cobo-Angel et al.
Scientific reports, 9(1), 14025-14025 (2019-10-03)
Group B Streptococcus (GBS), is a leading cause of neonatal death and an emerging pathogen in adults. Additionally, GBS is a bovine pathogen causing intramammary infections. The likelihood of GBS interspecies transmission is largely unknown. We explored the potential transmission
The Journal of Organic Chemistry, 59, 4535-4535 (1994)
Synlett, 431-431 (1994)
Tetrahedron, 50, 4557-4557 (1994)
Angela M Bernard et al.
Organic letters, 7(21), 4565-4568 (2005-10-08)
[reactions: see text] Regioselective synthesis of 2,4,5- or 3,4,5-trisubstituted 2,3-dihydrofurans has been realized by using donor-acceptor cyclopropanes or by a Corey ylide reaction with alpha-sulfenyl-, alpha-sulfinyl-, or alpha-sulfonylenones. The method allowed a straightforward synthesis of the natural product calyxolane B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service