H6515
L-Homoserine
Synonym(s):
(S)-2-Amino-4-hydroxybutyric acid, Hse
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
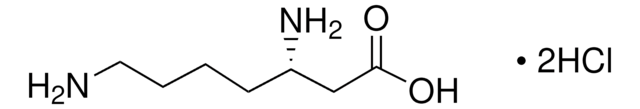
Linear Formula:
HOCH2CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
119.12
Beilstein:
1721681
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥98% (TLC)
form
powder
color
white to off-white
mp
203 °C (dec.) (lit.)
application(s)
cell analysis
detection
SMILES string
N[C@@H](CCO)C(O)=O
InChI
1S/C4H9NO3/c5-3(1-2-6)4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1
InChI key
UKAUYVFTDYCKQA-VKHMYHEASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
L-Homoserine is a variant of serine with an additional carbon on its side chain.
Application
L-Homoserine has been used as an internal standard for neurotransmitter analysis and amino acids quantification.
Biochem/physiol Actions
L-Homoserine is synthesized by deoxidation process, catalysed by homoserine dehydrogenase. This is one of the steps in the synthesis of L-threonine. The carbon flux in in bacteria such as E. coli is maintained by this reaction.
L-Homoserine is used in the biosynthesis of methionine, threonine and isoleucine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Neurotoxic effect of maneb in rats as studied by neurochemical and immunohistochemical parameters
Nielsen BS, et al.
Environmental Toxicology and Pharmacology, 21(3), 268-275 (2006)
Reprogramming Microbial Metabolic Pathways, 288-288 (2012)
Chiral recognition of non-natural ?-amino acids
Gal JF, et al.
International Journal of Mass Spectrometry, 222(1?3), 259-267 (2003)
D C Turnell et al.
Clinical chemistry, 28(3), 527-531 (1982-03-01)
This method for estimating clinically important amino acids in serum or urine within 40 min involves o-phthalaldehyde/2-mercaptoethanol derivatization and reversed-phase "high-pressure" liquid chromatography. Homocysteic acid is an internal standard, and homoserine and norvaline are reference peaks. For all the amino
Hsuan-Chen Wu et al.
Molecular systems biology, 9, 636-636 (2013-01-24)
Escherichia coli were genetically modified to enable programmed motility, sensing, and actuation based on the density of features on nearby surfaces. Then, based on calculated feature density, these cells expressed marker proteins to indicate phenotypic response. Specifically, site-specific synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service