PHR1026
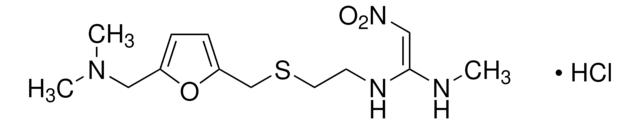
Ranitidine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
N, N Dimethyl-5-[2-(1-methylamine-2-nitrovinyl)-ethylthiomethyl]furfurylamine hydrochloride
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to BP 471
traceable to Ph. Eur. R0150000
traceable to USP 1598405
API family
ranitidine
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
Cl[H].CN\C(NCCSCc1ccc(CN(C)C)o1)=C\[N+]([O-])=O
InChI
1S/C13H22N4O3S.ClH/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3;/h4-5,9,14-15H,6-8,10H2,1-3H3;1H/b13-9-;
InChI key
GGWBHVILAJZWKJ-CHHCPSLASA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Biochem/physiol Actions
Analysis Note
Other Notes
Footnote
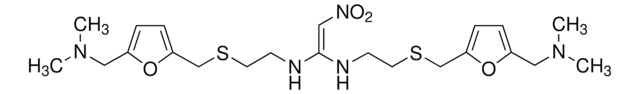
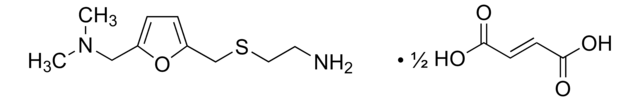
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service