76547
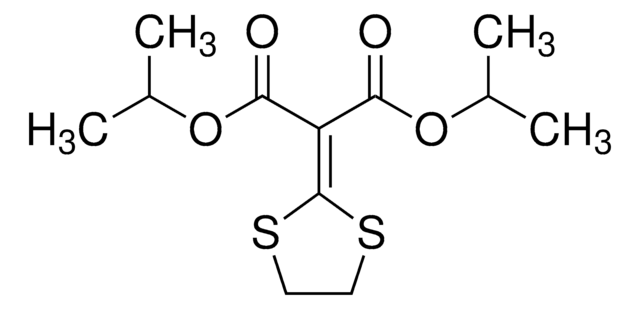
Isoprothiolane
PESTANAL®, analytical standard
Synonym(s):
Diisopropyl 2-(1,3-dithiolan-2-ylidene)malonate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H18O4S2
CAS Number:
Molecular Weight:
290.40
Beilstein:
2128528
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
Assay
≥98.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
SMILES string
CC(C)OC(=O)\C(C(=O)OC(C)C)=C1/SCCS1
InChI
1S/C12H18O4S2/c1-7(2)15-10(13)9(11(14)16-8(3)4)12-17-5-6-18-12/h7-8H,5-6H2,1-4H3
InChI key
UFHLMYOGRXOCSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Isoprothiolane is a systemic fungicide, which is used in controlling diseases rice stem rot, rice blast and Fusarium leaf spot on rice. Its mode of action basically involves the inhibition of penetration and elongation of infecting hyphae via inhibiting the formation of infecting peg or cellulose secretion.
Application
Isoprothiolane may be used as a reference standard in the determination of isoprothiolane in food samples using gas chromatography coupled with mass spectrometry (GC-MS).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Katamoto et al.
Nihon juigaku zasshi. The Japanese journal of veterinary science, 52(6), 1189-1197 (1990-12-01)
To study effects of isoprothiolane and phytosterol on dietary fat necrosis, 3 groups of rats were fed hardened-tallow (HT) diet. Two groups of rats received either isoprothiolane (50 mg/kg) or phytosterol (20 mg/kg) orally once a day consecutively for 10
H Okada et al.
The Journal of veterinary medical science, 61(5), 553-556 (1999-06-24)
This study was performed to investigate the effects of isoprothiolane on cell growth and the production of interleukin (IL)-1 and IL-6 by bovine mammary epithelial cells in vitro. Isoprothiolane increased proliferation of mammary epithelial cells in a dose-dependent manner at
Y Ishida et al.
Physiology & behavior, 60(2), 633-638 (1996-08-01)
Responses of three chemical senses, olfaction, taste, and pit organ sense, to three pesticides were studied electrophysiologically in carp, Cyprinus carpio. Only olfaction was responsive to the three pesticides at behavioral avoidance levels, which were determined in a previous study.
S S Chou et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 15(2), 135-146 (1980-01-01)
The fungicide isoprothiolane (diisopropyl 1,3-dithiolan-2-ylidenemalonate) decomposed slowly in deionized water under ultraviolet light or sunlight irradiation. Rice-paddy water greatly accelerated the photodegradation. This photosensitizing effect was comparable to that of 2% acetone. Soil extracts, rice-plant extracts, and chlorophylls showed little
Y Matsui et al.
Water science and technology : a journal of the International Association on Water Pollution Research, 56(1), 71-80 (2007-08-23)
Verification of a diffuse pollution model involves comparing results actually observed with those predicted by precise model inputs. Acquisition of precise model inputs is, however, problematic. In particular, when the target catchment is large and substantial estimation uncertainty exists, not
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service