182621
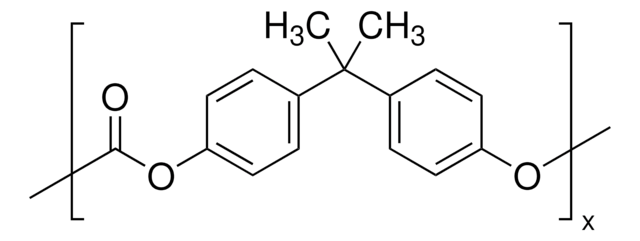
Poly(vinyl chloride)
analytical standard, average Mw 85,000 (Typical), average Mn 40,000 (Typical)
Synonym(s):
PVC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH2CHCl)n
CAS Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
form
powder
mol wt
average Mn 40,000 (Typical)
average Mw 85,000 (Typical)
technique(s)
gel permeation chromatography (GPC): suitable
density
1.4 g/mL at 25 °C (lit.)
format
neat
SMILES string
ClC=C
InChI
1S/C2H3Cl/c1-2-3/h2H,1H2
InChI key
BZHJMEDXRYGGRV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Poly(vinyl chloride) [PVC] is a polymer which is mostly prepared from vinyl chloride monomer. In most cases PVC is mixed with heat stabilizers, lubricants, plasticizers, fillers, and other additives.
Application
Labile chlorines of poly (vinyl chloride) was used to perform to graft copolymerize butyl acrylate and 2-ethyl hexyl acrylate using atom transfer radical polymerization method, followed by gel permeation chromatography (GPC) to identify the traces of graft copolymer samples.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Graft copolymerization of butyl acrylate and 2-ethyl hexyl acrylate from labile chlorines of poly (vinyl chloride) by atom transfer radical polymerization.
Bicak, Niyazi, and Mesut Ozlem.
Journal of Polymer Science Part A: Polymer Chemistry, 41.21, 3457-3462 (2003)
Poly (vinyl chloride)
Allsopp, Michael W., and Giovanni Vianello.
Ullmann's Encyclopedia of Industrial Chemistry, 28, 441-468 (1992)
T D Ambrisko et al.
British journal of anaesthesia, 110(2), 305-310 (2012-11-21)
In a previous study, the authors found a large bias (50%) for lithium (LiDCO) compared with thermodilution cardiac output measurement methods in ponies receiving i.v. infusions of xylazine, ketamine, and midazolam. This prompted the authors to examine the effect of
Hong Wang et al.
Environmental science & technology, 46(21), 11566-11574 (2012-10-11)
Opportunistic pathogens represent a unique challenge because they establish and grow within drinking water systems, yet the factors stimulating their proliferation are largely unknown. The purpose of this study was to examine the influence of pipe materials, disinfectant type, and
Saad S M Hassan et al.
Talanta, 103, 330-336 (2012-12-04)
New cost-effective potentiometric membrane sensors with cylindrical configuration responsive to ephedrine are described. The sensors setup is, based on the use of triacetyl-β-cyclodextrin [(triacetyl-β-CD)] as a neutral ionophore embedded in a plasticized poly (vinyl chloride) (PVC) matrix (sensor I) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service