I2800
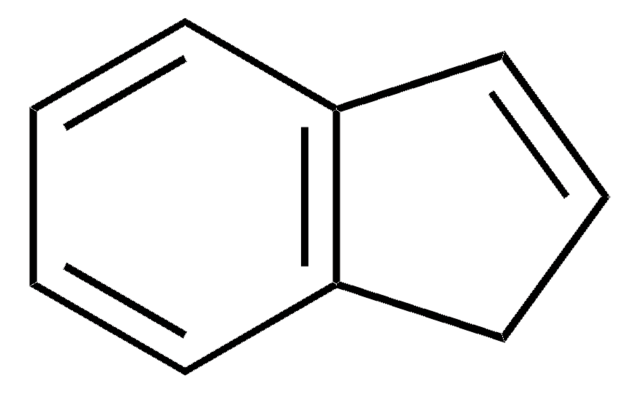
Indene
contains 50-100 ppm tert-butylcatechol as stabilizer, technical grade, ≥90%
Synonym(s):
Indonaphthene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8
CAS Number:
Molecular Weight:
116.16
Beilstein:
635873
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
≥90%
form
liquid
contains
50-100 ppm tert-butylcatechol as stabilizer
refractive index
n20/D 1.595 (lit.)
bp
181-182 °C (lit.)
mp
−5-−3 °C (lit.)
density
0.996 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=Cc2ccccc12
InChI
1S/C9H8/c1-2-5-9-7-3-6-8(9)4-1/h1-6H,7H2
InChI key
YBYIRNPNPLQARY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lucas J Gursky et al.
Applied microbiology and biotechnology, 85(4), 995-1004 (2009-07-02)
The styAB genes from Pseudomonas putida CA-3, which encode styrene monooxygenase, were subjected to three rounds of in vitro evolution using error-prone polymerase chain reaction with a view to improving the rate of styrene oxide and indene oxide formation. Improvements
Regioselective synthesis of indenols by rhodium-catalyzed C-H activation and carbocyclization of aryl ketones and alkynes.
Krishnamoorthy Muralirajan et al.
Angewandte Chemie (International ed. in English), 50(18), 4169-4172 (2011-03-31)
Two-step synthesis of stable dioxadicarbaporphyrins from bis(3-indenyl)methane.
Timothy D Lash et al.
Angewandte Chemie (International ed. in English), 51(43), 10871-10875 (2012-09-25)
Adam C Glass et al.
Organic letters, 10(21), 4855-4857 (2008-10-07)
A new methodology for the preparation of substituted naphthalenes starting from readily available indenones, organometal reagents, and trimethylsilyldiazomethane via a catalytic rearrangement process is described. Hindered biaryl naphthalenes, including triortho-substituted biaryls, can be accessed through our method. Our results are
Cobalt-catalyzed regioselective synthesis of indenamine from o-iodobenzaldimine and alkyne: intriguing difference to the nickel-catalyzed reaction.
Chuan-Che Liu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(31), 9503-9506 (2008-09-24)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service