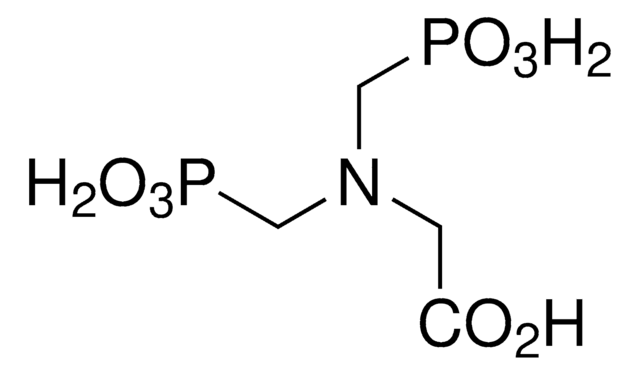
I0631
Imidodiphosphate sodium salt
≥97%
Synonym(s):
Tetrasodium imidodiphosphate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HNO6P2Na4
CAS Number:
Molecular Weight:
264.92
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
storage temp.
2-8°C
SMILES string
O=P([O-])([O-])NP([O-])([O-])=O.[Na+].[Na+].[Na+].[Na+]
InChI
1S/H5NO6P2.4Na/c2-8(3,4)1-9(5,6)7;;;;/h(H5,1,2,3,4,5,6,7);;;;/q;4*+1/p-4
InChI key
KDZOOFFPQVQSQG-UHFFFAOYSA-J
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N F Zakirova et al.
Bioorganicheskaia khimiia, 31(1), 96-102 (2005-03-25)
The preparation conditions for dichlorophosphinylphosphorimidic trichloride were optimized. It was used in the synthesis of esters of imidodiphosphoric acid. The interaction of the trichloride with amines resulted in the corresponding amidodiphosphates rather than in the expected amides of imidodiphosphoric acid.
T Rozovskaya et al.
FEBS letters, 247(2), 289-292 (1989-04-24)
It is demonstrated here that rat liver DNA polymerase beta catalyzes the pyrophosphorolysis reaction with pyrophosphate (PPi) and its analogues. The substrate specificity of the PPi-binding site of several DNA polymerases was investigated. It was discovered that the ability of
Highly efficient green and blue-green phosphorescent OLEDs based on iridium complexes with the tetraphenylimidodiphosphinate ligand.
Yu-Cheng Zhu et al.
Advanced materials (Deerfield Beach, Fla.), 23(35), 4041-4046 (2011-07-30)
Andrew P Bassett et al.
Inorganic chemistry, 44(18), 6140-6142 (2005-08-30)
Near-infrared emitting complexes of Nd(III), Er(III), and Yb(III) based on hexacoordinate lanthanide ions with an aryl functionalized imidodiphosphinate ligand, tpip, have been synthesized and fully characterized. Three tpip ligands form a shell around the lanthanide with the ligand coordinating via
Jae Park et al.
Molecular and cellular biochemistry, 283(1-2), 11-21 (2006-01-31)
The enzyme adenosine kinase (AK) plays a central role in regulating the intracellular and interstitial concentration of the purine nucleoside adenosine (Ado). In view of the beneficial effects of Ado in protecting tissues from ischemia and other stresses, there is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service