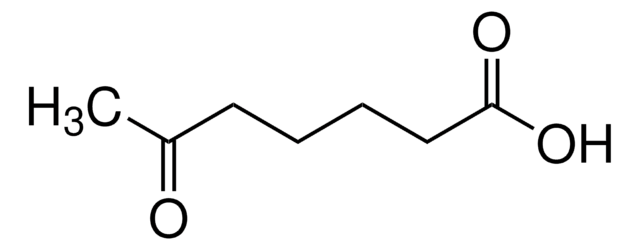
A13204
4-Acetylbutyric acid
97%
Synonym(s):
5-Ketohexanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CO(CH2)3CO2H
CAS Number:
Molecular Weight:
130.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.4451 (lit.)
bp
274-275 °C (lit.)
mp
13-14 °C (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
CC(=O)CCCC(O)=O
InChI
1S/C6H10O3/c1-5(7)3-2-4-6(8)9/h2-4H2,1H3,(H,8,9)
InChI key
MGTZCLMLSSAXLD-UHFFFAOYSA-N
Application
4-Acetylbutyric acid may be used in the preparation of the following compounds:
- 5-hydroxyhexanoic acid
- 6-methyl1-3,4-dihydro-pyran-2-one, precursor for 5-acetyl-tetrahydro-2-(3H)-furanone
- substituted N-aminolactams
- (±)-5-methyl-δ-valerolactone
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of 5-hydroxyhexanoic acid, 4-hydroxyheptanoic acid and 4-hydroxyoctanoic acid as new constituents of bacterial polyhydroxyalkanoic acids.
Valentin HE, et al.
Applied Microbiology and Biotechnology, 46(3), 261-267 (1996)
(2S, 6S, 8S)-2, 8-Dimethyl-1, 7-dioxaspiro [5.5] undecane: A natural spiroacetal lacking anomeric stabilisation
Chen J, et al.
Tetrahedron Asymmetry, 6.4, 967- 972 (1995)
Proline-like β-turn mimics accessed via Ugi reaction involving monoprotected hydrazines.
Krasavin M, et al.
Tetrahedron Letters, 51(10), 1367-1370 (2010)
A versatile and concise route to functionally substituted ?-butyrolactones and spiro-XXX-butyrolactones (lactone annelation)
Mandal AK and Jawalkar DG
Tetrahedron Letters, 27.1, 99-100 (1986)
Jie Yao et al.
Water science and technology : a journal of the International Association on Water Pollution Research, 79(11), 2195-2202 (2019-07-19)
The monoterpene alcohol α-terpineol is extensively used as the foaming agent in mineral processing and can be released to environment along with the wastewater. This study evaluated the feasibility of eliminating α-terpineol in water by ultraviolet irradiation (UV) in combination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service