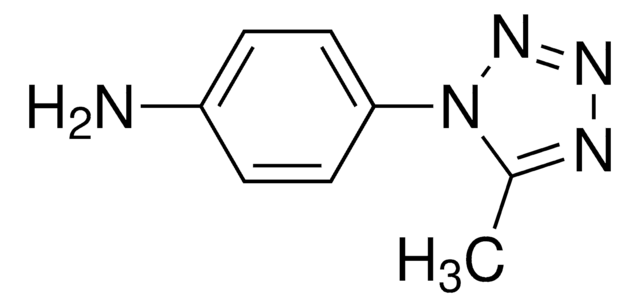
927570
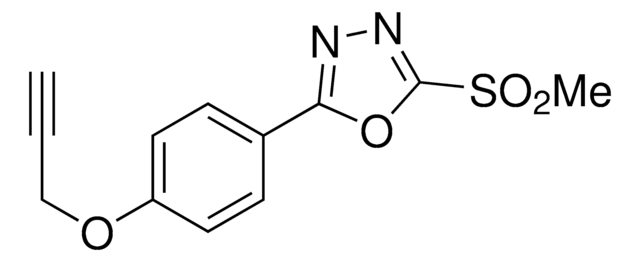
MST-alkyne
≥95%
Synonym(s):
5-(Methylsulfonyl)-1-(4-(prop-2-yn-1-yloxy)phenyl)-1H-tetrazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H10N4O3S
CAS Number:
Molecular Weight:
278.29
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Application
MST-alkyne is a probe that can be used to label cysteines through nucleophilic aromatic substitution. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications . The method uses light or heavy labeling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis . Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow .
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Isotopically Labeled Desthiobiotin Azide (isoDTB) Tags Enable Global Profiling of the Bacterial Cysteinome
Zanon, et al
Angewandte Chemie (International Edition in English), 2829-2836 (2020)
Profiling the proteome-wide selectivity of diverse electrophiles
Zanon, et al
ChemRxiv : the preprint server for chemistry (2021)
Eranthie Weerapana et al.
Nature, 468(7325), 790-795 (2010-11-19)
Cysteine is the most intrinsically nucleophilic amino acid in proteins, where its reactivity is tuned to perform diverse biochemical functions. The absence of a consensus sequence that defines functional cysteines in proteins has hindered their discovery and characterization. Here we
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service