All Photos(1)
About This Item
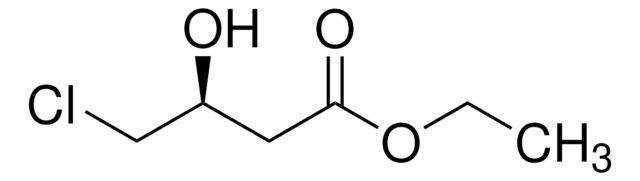
Linear Formula:
CH3CH(OH)CH2CO2C2H5
CAS Number:
Molecular Weight:
132.16
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
optical activity
[α]20/D −46°, c = 1 in chloroform
optical purity
ee: 99% (GLC)
refractive index
n20/D 1.42 (lit.)
bp
75-76 °C/12 mmHg (lit.)
density
1.017 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C[C@@H](C)O
InChI
1S/C6H12O3/c1-3-9-6(8)4-5(2)7/h5,7H,3-4H2,1-2H3/t5-/m1/s1
InChI key
OMSUIQOIVADKIM-RXMQYKEDSA-N
Related Categories
General description
Ethyl (R)-(-)-3-hydroxybutyrate is a chiral building block for the preparation of bioactive compounds used in the pharmaceutical industry. It is formed during the hydrolysis of poly-3-hydroxybutyrate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A two-step enzymatic resolution process for large-scale production of (S)-and (R)-ethyl-3-hydroxybutyrate.
Fishman A, et al.
Biotechnology and Bioengineering, 74(3), 256-263 (2001)
Young-Min Han et al.
Molecular cell, 71(6), 1064-1078 (2018-09-11)
β-hydroxybutyrate (β-HB) elevation during fasting or caloric restriction is believed to induce anti-aging effects and alleviate aging-related neurodegeneration. However, whether β-HB alters the senescence pathway in vascular cells remains unknown. Here we report that β-HB promotes vascular cell quiescence, which
Xiao-Hong Chen et al.
PloS one, 9(4), e94543-e94543 (2014-04-18)
A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The enzyme showed a homotetrameric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)
