120227
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
95%
Synonym(s):
Hantzsch ester
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C13H19NO4
CAS Number:
Molecular Weight:
253.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
178-183 °C (lit.)
solubility
organic solvents: soluble
SMILES string
CCOC(=O)C1=C(C)NC(C)=C(C1)C(=O)OCC
InChI
1S/C13H19NO4/c1-5-17-12(15)10-7-11(13(16)18-6-2)9(4)14-8(10)3/h14H,5-7H2,1-4H3
InChI key
LJXTYJXBORAIHX-UHFFFAOYSA-N
Related Categories
General description
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate is often used as a building block in organic synthesis for the preparation of various biologically active compounds.
Application
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (DTP) was used to study the mechanism of electrochemical oxidation of DTP in ethanol/water solutions on a glassy carbon electrode.
Used as a hydrogen source in organocatalytic reductive amination and conjugate reduction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jing Zhang et al.
iScience, 23(1), 100755-100755 (2019-12-31)
The alkoxyl radical is an essential reactive intermediate in mechanistic studies and organic synthesis with hydrogen atom transfer (HAT) reactivity. However, compared with intramolecular 1,5-HAT or intermolecular HAT of alkoxyl radicals, the intramolecular 1,2-HAT reactivity has been limited to theoretical
R Nosal et al.
Bratislavske lekarske listy, 102(10), 447-453 (2002-01-23)
Metabolites of arachidonic acid are important regulatory substances in blood platelets. They participate in platelet adhesion and aggregation, and pharmacological intervention with arachidonate cascade is widely used in therapy of hyperactive platelets and in the prevention of thromboembolic complications. To
V Z Gorkin et al.
Vestnik Rossiiskoi akademii meditsinskikh nauk, (2)(2), 12-17 (1995-01-01)
The art-of-the-state and possible perspectives for studies of the properties of amine oxidases which are medically significant are briefly outlined. Due to the studies conducted at the Research Institute of Biomedical Chemistry of the Russian Academy of Medical Sciences, the
N A Basova et al.
Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova, 88(5), 650-657 (2002-07-26)
The main concern of this work was to examine the relation between altered antioxidant status on the one hand and increase in L-tryptophan absorption in the small intestine in order to bring further information regarding to possible role of vitamin
V F Dubrovskaja et al.
Radiobiologia, radiotherapia, 31(1), 79-84 (1990-01-01)
Female mice (CBA X C57 Bl) F1 were exposed a single total-body gamma-irradiation with a dose efficiency of 6.5 Gy/min with doses of 2.5-7.5 Gy in air or inhaling a gas mixture of 6-6.5% oxygen and 94-93% nitrogen. All naturally
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service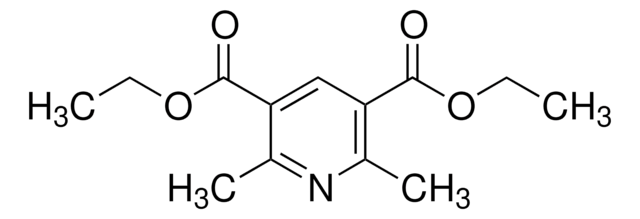








![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) sublimed grade](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)