63102
Magnesium perchlorate
puriss., free-flowing powder, ≥99.0% (calc. based on dry substance, KT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Mg(ClO4)2
CAS Number:
Molecular Weight:
223.21
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
Assay:
≥99.0% (calc. based on dry substance, KT)
form:
free flowing powder
Recommended Products
grade
puriss.
Quality Level
Assay
≥99.0% (calc. based on dry substance, KT)
form
free flowing powder
quality
free-flowing powder
impurities
≤8% water
storage temp.
room temp
SMILES string
[Mg++].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/2ClHO4.Mg/c2*2-1(3,4)5;/h2*(H,2,3,4,5);/q;;+2/p-2
InChI key
MPCRDALPQLDDFX-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Magnesium perchlorate (Mg(ClO4)2) is widely used as drying agent for gases. It can remove water from gases (with no organic contaminants) at the rate of 0.001mgwater/l.
Application
Magnesium perchlorate (Mg(ClO4)2) may be employed as a catalyst in the following studies:
- Preparation of a-aminophosphonates.
- Enantioselective Diels-Alder reaction between cyclopentadiene and 3-acryloyl-1,3-oxazolin-2-one.
- Preparation of imines and phenylhydrazones.
- Protection of alcohols in the form of t-butyl ethers.
filler for drying tubes (especially for elemental analysis)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The first enantioselective synthesis of both Diels-Alder enantiomers with the same bis (oxazoline)-magnesium perchlorate chiral catalyst.
Desimoni G, et al.
Tetrahedron Letters, 37(17), 3027-3030 (1996)
Magnesium perchlorate as an efficient catalyst for the synthesis of imines and phenylhydrazones.
Chakraborti AK, et al.
Tetrahedron Letters, 45(41), 7641-7644 (2004)
Eagleson M.
Concise Encyclopedia Chemistry, 343-343 (1994)
T Theophanides
Magnesium research, 9(4), 259-262 (1996-12-01)
The interaction of Mg2+ cations in biological systems is studied by using nucleic acid bases as the biological system. Magnesium salts, such as, MgCl2 6H2O, MgSO4. 7H2O and Mg(ClO4). XH2O have been employed in order to compare their complexation with
K Miyake et al.
Photochemistry and photobiology, 58(5), 631-636 (1993-11-01)
We have investigated the photosensitized monomerization of the cis,syn-cyclobutane dimer of 1,3-dimethylthymine using riboflavin tetraacetate and a 5-deazaflavin derivative as photosensitizer. Although little monomerization of the dimer is induced by photoexcitation of the flavins in the absence of any additives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
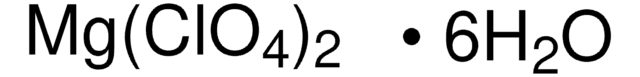

![Magnesium perchlorate hydrate [about 83% Mg(ClO₄)₂] for analysis EMSURE®](/deepweb/assets/sigmaaldrich/product/images/416/076/53566e58-8fe6-49fb-904e-a2cea87aa289/640/53566e58-8fe6-49fb-904e-a2cea87aa289.jpg)
![Magnesium perchlorate hydrate [about 83% Mg(ClO₄)₂], desiccant, about 1-4 mm](/deepweb/assets/sigmaaldrich/product/images/443/694/23ff72e8-a615-4835-b5af-31f59eb41e91/640/23ff72e8-a615-4835-b5af-31f59eb41e91.jpg)

![Magnesium perchlorate hydrate [about 83% Mg(ClO₄)₂] for elemental analysis](/deepweb/assets/sigmaaldrich/product/structures/316/217/33fdb42c-cc3d-4f19-9710-5872b6e25bdd/640/33fdb42c-cc3d-4f19-9710-5872b6e25bdd.png)







