777293
2-{[7-(5-N,N-Ditolylaminothiophen-2-yl)-2,1,3-benzothiadiazol-4-yl]methylene}malononitrile
99% (HPLC)
Synonym(s):
2-[[7-[5-[Bis(4-methylphenyl)amino]-2-thienyl]-2,1,3-benzothiadiazol-4-yl]methylene]propanedinitrile, DTDCTB
About This Item
Recommended Products
Assay
99% (HPLC)
form
powder
mp
230-235 °C
transition temp
Tm 233 °C
λmax
662-664 nm in dichloromethane
SMILES string
CC(C=C1)=CC=C1N(C2=CC=C(C)C=C2)C3=CC=C(C4=CC=C(C=C(C#N)C#N)C5=NSN=C54)S3
InChI
1S/C28H19N5S2/c1-18-3-8-22(9-4-18)33(23-10-5-19(2)6-11-23)26-14-13-25(34-26)24-12-7-21(15-20(16-29)17-30)27-28(24)32-35-31-27/h3-15H,1-2H3
InChI key
BCJCBXQJAANTJL-UHFFFAOYSA-N
General description
MoO3 (30nm) / DTDCTB (7nm) / DTDCTB:C60/C70 (40nm) / C60/C70 (7nm) / BCP (10nm) / Ag
Device performance:
- JSC = 14.68 mA/cm2
- VOC = 0.8 V
- FF = 0.5
- PCE = 5.81%
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
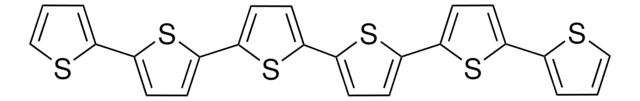
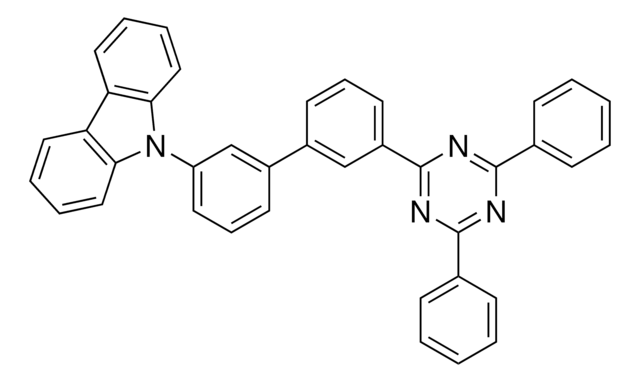
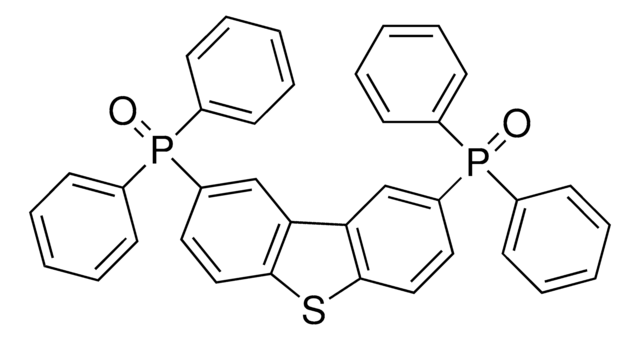
Customers Also Viewed
Articles
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service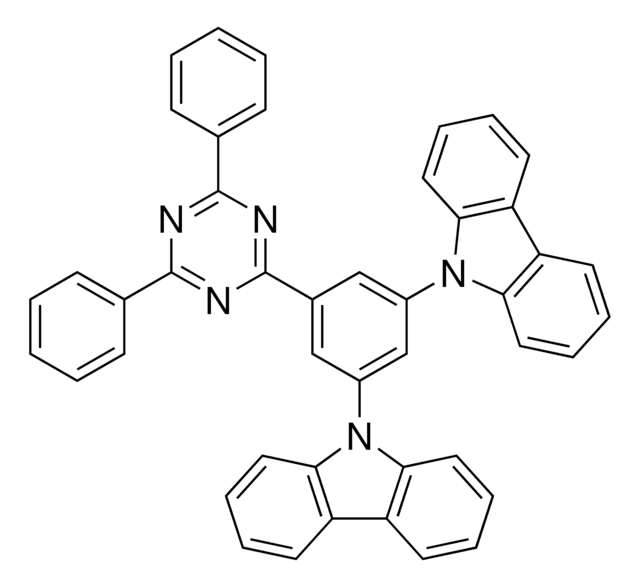
iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/309/053/0823f035-245c-433d-b033-2eca2d931c67/640/0823f035-245c-433d-b033-2eca2d931c67.png)
![3,6-Bis(5-bromo-2-thienyl)-2,5-bis(2-hexyldecyl)-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione 98%](/deepweb/assets/sigmaaldrich/product/structures/128/499/590a62c1-529b-42e2-96df-25659ec8c9e0/640/590a62c1-529b-42e2-96df-25659ec8c9e0.png)


![2,4-Bis[4-(N,N-diphenylamino)-2,6-dihydroxyphenyl]squaraine 98%](/deepweb/assets/sigmaaldrich/product/structures/303/054/d8b9c845-3623-4f5a-8a30-ab6731034171/640/d8b9c845-3623-4f5a-8a30-ab6731034171.png)