All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6O4
CAS Number:
Molecular Weight:
190.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
202-205 °C (lit.)
SMILES string
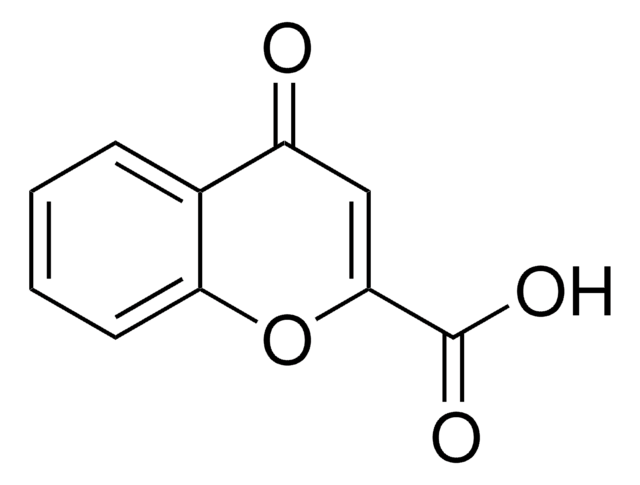
OC(=O)C1=COc2ccccc2C1=O
InChI
1S/C10H6O4/c11-9-6-3-1-2-4-8(6)14-5-7(9)10(12)13/h1-5H,(H,12,13)
InChI key
PCIITXGDSHXTSN-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
Chromone-3-carboxylic acid is a chromone derivative. Its potential as an antioxidant in charge transfer (CT) processes has been assessed by surface-enhanced Raman scattering (SERS) analysis of chromone 3-carboxylic acid adsorbed on silver colloids.
Application
Chromone-3-carboxylic acid may be used in the preparation of:
- chromane-2,4-diones
- chromone-3-carboxamides
- 5-(2-hydroxyphenyl)isoxazole
- chromone-2-carboxamides
- chromone-2-carboxamido-3-esters
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Conjugate addition of isocyanides to chromone 3-carboxylic acid: an efficient one-pot synthesis of chroman-4-one 2-carboxamides.
Neo AG, et al.
Organic & Biomolecular Chemistry, 10(17), 3406-3416 (2012)
Chromone-3-carboxylic acid as a potential electron scavenger: a surface-enhanced Raman scattering study.
Machado NFL, et al.
Physical Chemistry Chemical Physics, 13(3), 1012-1018 (2011)
Synthesis of [1] benzopyrano [3,4-d] isoxazol-4-ones from 2-substituted chromone-3-carboxylic esters. A reinvestigation of the reaction of 3-acyl-4-hydroxycoumarins with hydroxylamine. Synthesis of 4-(2-hydroxybenzoyl) isoxazol-5-ones.
Chantegrel B, et al.
The Journal of Organic Chemistry, 49(23), 4419-4424 (1984)
F Cagide et al.
Chemical communications (Cambridge, England), 51(14), 2832-2835 (2015-01-13)
The discovery of potent and selective monoamine oxidase-B inhibitors for the management of neurodegenerative diseases such as Alzheimer's and Parkinson's diseases is still a challenging endeavor. Herein, we report the discovery of two new classes of potent and selective MAO-B
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service