I601
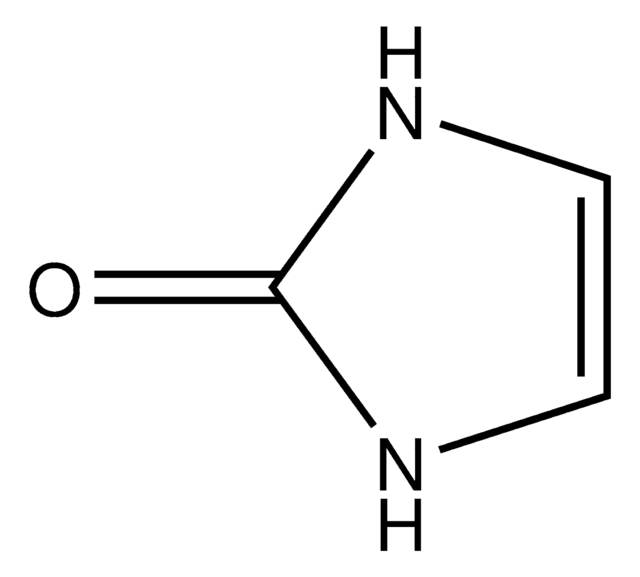
2-Imidazolidone
96%
Synonym(s):
2-Oxoimidazolidine, N,N′-Ethyleneurea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6N2O
CAS Number:
Molecular Weight:
86.09
Beilstein:
106252
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
129-132 °C (lit.)
SMILES string
O=C1NCCN1
InChI
1S/C3H6N2O/c6-3-4-1-2-5-3/h1-2H2,(H2,4,5,6)
InChI key
YAMHXTCMCPHKLN-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
Reactant for:
- Chiral microporous materials from achiral precursors
- Aryl and heteroaryl N-acylureas via microwave-assisted palladium-catalyzed carbonylation
- A highly water-soluble peptide based human neutrophil elastase inhibitor
- Heterocycles by cyanoacetylation for antimicrobial use
Reactant for:
- Pd-catalyzed C-N bond formation with heteroaromatic tosylates
- Oxidative amidation of activated alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT RE 2
Target Organs
Thyroid
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mette L H Mantel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(18), 5437-5442 (2010-04-09)
A protocol for the palladium(0)-catalyzed amidation of heteroaromatic tosylates was successfully developed. The methodology proved to be effective for a variety of heteroaryl tosylates including the pyridine, pyrimidine, quinoline and quinoxaline ring systems. Successful carbon-nitrogen bond formation with these heteroaryl
Rafael Bautista et al.
The Journal of organic chemistry, 76(19), 7901-7911 (2011-08-17)
An efficient and versatile synthesis of novel exo-imidazolidin-2-one dienes is described. This involves the base-assisted condensation/cyclization cascade reaction of the monoimino derivatives of diacetyl with a series of isocyanates. This methodology enables preparation of symmetrical dienes, as long as the
J F Albuquerque et al.
Annales pharmaceutiques francaises, 53(5), 209-214 (1995-01-01)
The synthesis and the physico-chemical properties of four 3-(4-bromophenacyl)-5-arylidene-thiazolidine-2,4-diones, two 3-(4-bromobenzyl)-5-arylidene-thiazolidine-2,4-diones and seven 3-(4-chlorobenzyl)-5-arylidene-4-thio-imidazolidine-2-ones were described. These products were synthetized by the aldolisation-crotonisation reaction between aromatic aldehydes and substituted thiazolidinediones or thio-imidazolidinones.
[Preliminary pharmacological evaluation of new imidazolidinone-2, ethylenediamine and imidazoline derivatives].
E Jagiełło-Wójtowicz et al.
Acta poloniae pharmaceutica, 41(4), 495-504 (1984-01-01)
Alaa A-M Abdel-Aziz et al.
Bioorganic & medicinal chemistry letters, 22(5), 2008-2014 (2012-02-10)
Novel series of 1-(arenesulfonyl)imidazolidin-2-one (3a-i) and 1,3-bis(arenesulfonyl)imidazolidin-2-one (5a-i) have been synthesized and tested for their antitumor activity against 60 tumor cell lines taken from nine different organs. A significant inhibition for cancer cells was observed with series 5a-i compounds compared
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service