274852
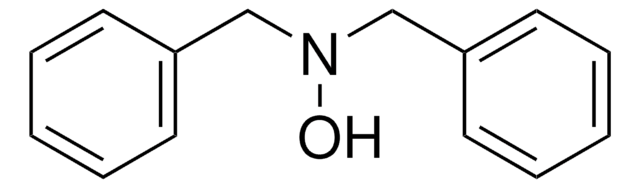
N-Benzoyl-N-phenylhydroxylamine
98%
Synonym(s):
N-Hydroxy-N-phenylbenzamide, N-Phenylbenzohydroxamic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CON(OH)C6H5
CAS Number:
Molecular Weight:
213.23
Beilstein:
2212449
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
118-120 °C (lit.)
storage temp.
2-8°C
SMILES string
ON(c1ccccc1)C(=O)c2ccccc2
InChI
1S/C13H11NO2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,16H
InChI key
YLYIXDZITBMCIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The mechanism of the reaction of N-benzoyl-N-phenylhydroxylamine with vanadium(IV) was studied.
Application
N-Benzoyl-N-phenylhydroxylamine was used as a complexing agent for studying the dispersive liquid-liquid microextraction based on solidification of floating organic drop (DLLME-SFO) behavior of vanadium (V). It was used as an extractant in the determination of beryllium in natural waters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction mechanism of N-benzoyl-N-phenylhydroxylamine with vanadium(IV) in the weakly acidic medium.
Z Nan
Talanta, 52(5), 785-789 (2008-10-31)
The N-benzoyl-N-phenylhydroxylamine(BPHA)-V(IV) system, if kept de-aerated, gives no color reaction. In an open vessel a color reaction does take place. This reaction was studied in solution by spectrophotometry, and the product prepared as solid by scanning thermal analysis, X-ray photoelectron
L C Robles et al.
The Analyst, 116(7), 735-737 (1991-07-01)
A procedure for the determination of beryllium in natural waters is proposed. A solvent extraction step was performed in order to overcome interferences and isolate beryllium before it was atomized by direct nebulization of the organic phase in a dinitrogen
Xiaoshan Huang et al.
International journal of analytical chemistry, 2018, 8045324-8045324 (2018-08-30)
A new sensitive method for antimony (III) determination by graphite furnace atomic absorption spectrometry (GFAAS) has been developed by using N-benzoyl-N-phenylhydroxylamine (BPHA) and 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) single drop microextraction. The single drop microextraction (SDMM) system is more competitive compared with
Tahereh Asadollahi et al.
Talanta, 82(1), 208-212 (2010-08-06)
A novel dispersive liquid-liquid microextraction based on solidification of floating organic drop (DLLME-SFO) for separation/preconcentration of ultra trace amount of vanadium and its determination with the electrothermal atomic absorption spectrometry (ETAAS) was developed. The DLLME-SFO behavior of vanadium (V) using
Jie Li et al.
Talanta, 81(3), 954-958 (2010-03-20)
A simple and rapid analytical method for determining the concentration of rhenium in molybdenite for Re-Os dating was developed. The method used isotope dilution-inductively coupled plasma-mass spectrometry (ID-ICP-MS) after the removal of major matrix elements (e.g., Mo, Fe, and W)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service