All Photos(1)
About This Item
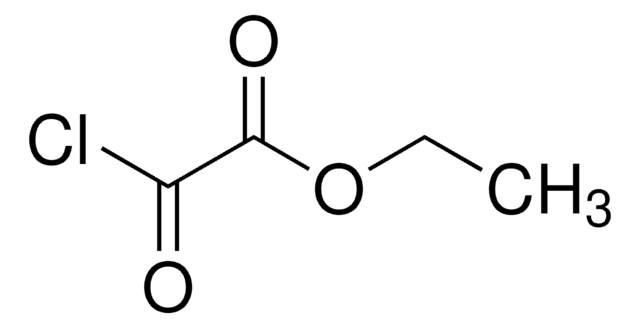
Linear Formula:
CH3CHClCOCH3
CAS Number:
Molecular Weight:
106.55
Beilstein:
385637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
114-117 °C (lit.)
density
1.055 g/mL at 25 °C (lit.)
SMILES string
CC(Cl)C(C)=O
InChI
1S/C4H7ClO/c1-3(5)4(2)6/h3H,1-2H3
InChI key
OIMRLHCSLQUXLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Chloro-2-butanone reacts with 1,4-dianion of acetophenone N-ethoxycarbonylhydrazone to yield pyrazoline derivatives.
Application
3-Chloro-2-butanone was used in production of chiral alcohols from acetophenone derivatives, β-ketoesters and N-Boc-3-pyrrolidinone by recombinant E. coli cells. It was used in the synthesis of carbene precursor, 3-aryl-4,5-dimethylthiazolium chloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction of α-chloroketones with 1, 4-dianion of acetophenone n-ethoxy-carbonylhydrazone.
Matsumura N, et al.
Tetrahedron Letters, 25(40), 4529-4532 (1984)
Nobuya Itoh et al.
European journal of biochemistry, 269(9), 2394-2402 (2002-05-03)
Phenylacetaldehyde reductase (PAR) produced by styrene-assimilating Corynebacterium strain ST-10 was used to synthesize chiral alcohols. This enzyme with a broad substrate range reduced various prochiral aromatic ketones and beta-ketoesters to yield optically active secondary alcohols with an enantiomeric purity of
Georgios C Vougioukalakis et al.
Journal of the American Chemical Society, 130(7), 2234-2245 (2008-01-29)
A new family of ruthenium-based olefin metathesis catalysts bearing a series of thiazole-2-ylidene ligands has been prepared. These complexes are readily accessible in one step from commercially available (PCy3)2Cl2Ru=CHPh or (PCy3)Cl2Ru=CH(o-iPrO-Ph) and have been fully characterized. The X-ray crystal structures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service