163244
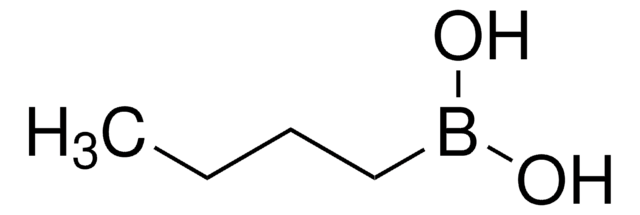
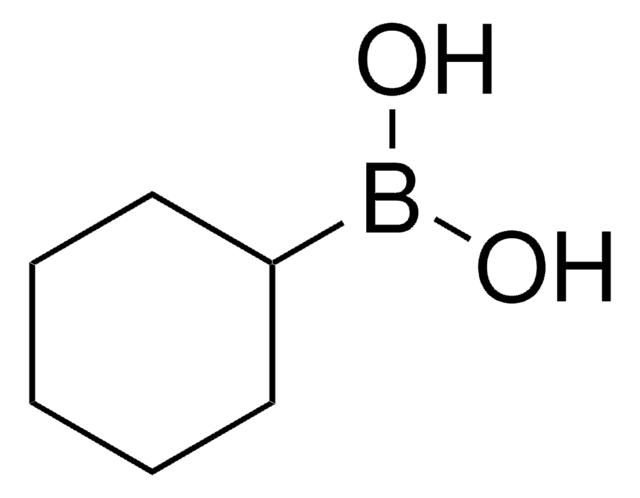
Butylboronic acid
97%
Synonym(s):
1-Butaneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3(CH2)3B(OH)2
CAS Number:
Molecular Weight:
101.94
Beilstein:
1733489
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
90-92 °C (lit.)
SMILES string
CCCCB(O)O
InChI
1S/C4H11BO2/c1-2-3-4-5(6)7/h6-7H,2-4H2,1H3
InChI key
QPKFVRWIISEVCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
A precursor to unsymmetric borinic acids, inhibitors of serine proteases. Reagent used to prepare chiral oxazaborolidines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 35, 4419-4419 (1994)
Journal of the American Chemical Society, 116, 3151-3151 (1994)
L C Taylor et al.
Rapid communications in mass spectrometry : RCM, 8(3), 265-273 (1994-03-01)
Liquid chromatography (LC) combined with atmospheric pressure chemical ionization mass spectrometry was used to identify phase I and II metabolites of the drug BW 1370U87 in dog and human urine. Additional analysis of individual high-performance liquid chromatograph fractions collected from
L D Sutton et al.
Biochemical and biophysical research communications, 134(1), 386-392 (1986-01-14)
The cholesterol esterase and lipoprotein lipase catalyzed hydrolyses of the water-soluble substrate p-nitrophenyl butyrate are competitively inhibited by butaneboronic acid and phenylboronic acid. Phenyl-n-butylborinic acid has been synthesized and characterized as an ultrapotent transition state analog inhibitor: Ki = 2.9
Alicja J Copik et al.
Inorganic chemistry, 44(5), 1160-1162 (2005-03-01)
Metalloproteases utilize their active site divalent metal ions to generate a nucleophilic water/hydroxide. For methionine aminopeptidases (MetAPs), the exact location of this nucleophile, as well as of the substrate, with respect to the active site metal ion is unknown. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service