137766
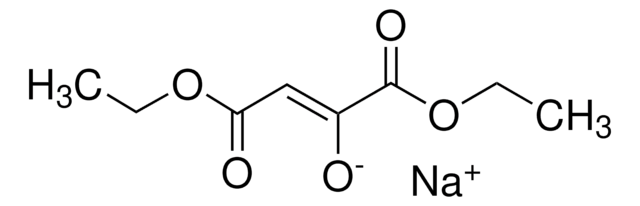
Diethyl oxalpropionate
≥95%
Synonym(s):
Diethyl 2-methyl-3-oxosuccinate, Methyloxo-butanedioic acid diethyl ester, NSC 33946
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOCH(CH3)COCOOC2H5
CAS Number:
Molecular Weight:
202.20
Beilstein:
1783697
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
refractive index
n20/D 1.432 (lit.)
bp
138 °C/23 mmHg (lit.)
density
1.073 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
CCOC(=O)C(C)C(=O)C(=O)OCC
InChI
1S/C9H14O5/c1-4-13-8(11)6(3)7(10)9(12)14-5-2/h6H,4-5H2,1-3H3
InChI key
OQOCQBJWOCRPQY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Diethyl oxalpropionate was used in the synthesis of 2-hydroxy-3-methylsuccinic acid. It was used as starting reagent in the synthesis of racemic 4-alkyloxycarbonyl-3,3-dimethyl-2-oxetanones and 4-quinolinone 2-carboxylic acid.
Reactant involved in:
- Synthesis of anticancer and antiviral agents
- Oxidation by organohypervalent iodine reagent
- Synthesis of monomers for preparation of functional polyesters
- Synthesis of calpain inhibitors
- Preparation of human A2A receptor antagonists
- Structural studies of dihydropteroate synthase inhibitors
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dong Hyuk Nam et al.
Bioorganic & medicinal chemistry letters, 18(1), 205-209 (2007-11-21)
Calpains are involved in a variety of calcium-regulated cellular processes, such as signal transduction, cell proliferation, differentiation, and apoptosis. Excessive calpain activation contributes to serious cellular damage and has been reported in many pathological conditions. 4-Quinolinone 2-carboxamide derivatives were prepared
Leanne A Pearson et al.
The Journal of biological chemistry, 282(7), 4681-4692 (2006-12-05)
The cyanobacterium Microcystis aeruginosa is widely known for its production of the potent hepatotoxin microcystin. This cyclic heptapeptide is synthesized non-ribosomally by the thio-template function of a large modular enzyme complex encoded within the 55-kb microcystin synthetase gene (mcy) cluster.
Synthesis of New Homopolyester and Copolyesters by Anionic Ring-opening Polymerization of a, a', ?-Trisubstituted ?-Lactones.
Barbaud C, et al.
Macromolecular Chemistry and Physics, 205(2), 199-207 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service