123617
Benzo[h]quinoline
97%
Synonym(s):
1-Naphthoquinoline, 7,8-Benzoquinoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H9N
CAS Number:
Molecular Weight:
179.22
Beilstein:
120249
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
338 °C/719 mmHg (lit.)
mp
48-50 °C (lit.)
SMILES string
c1ccc2c(c1)ccc3cccnc23
InChI
1S/C13H9N/c1-2-6-12-10(4-1)7-8-11-5-3-9-14-13(11)12/h1-9H
InChI key
WZJYKHNJTSNBHV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Benzo[h]quinoline was used to study the mutagenic activities of benzo[f]quinoline, benzo[h]quinolone and a number of their derivatives in strain TA 100 of Salmonella typhimurium. It was used in determination of nitrogen-containing polynuclear aromatic hydrocarbons in the gaseous products of the thermal degradation of polymers by HPLC- fluorescence detection. It was used as starting reagent for the synthesis of osmium and ruthenium complexes containing an N-heterocyclic carbene ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ping-Chieh Hsieh et al.
Environmental toxicology and chemistry, 29(8), 1696-1702 (2010-09-08)
The binding constant (K(DOC)) between humic acid and the nitrogen-containing polycyclic aromatic compound (N-PAC), benzo[h]quinoline, was measured at varying pH levels using fluorescence quenching (FQ). Because fluorescence characteristics of benzo[h]quinoline change with pH, determination required two optimum sets of excitation
S Kumar et al.
Cancer research, 49(1), 20-24 (1989-01-01)
The mutagenic activities of benzo[f]quinoline, benzo[h]quinoline, and a number of their derivatives, including dihydrodiols, K-region oxides, diol epoxides, and tetrahydroepoxides, were assessed in strain TA 100 of Salmonella typhimurium. The dihydrodiol derivatives of benzo[f]quinoline and benzo[h]quinoline were also tested for
Osmium and Ruthenium Complexes Containing an N-Heterocyclic Carbene Ligand Derived from Benzo [h] quinoline.
Esteruelas MA, et al
Organometallics, 26(21), 5239-5245 (2007)
Hanumantharao Paritala et al.
Bioorganic & medicinal chemistry letters, 19(6), 1584-1587 (2009-02-27)
G-quadruplexes are unusual structures formed from guanine-rich sequences of nucleic acids. G-quadruplexes have been postulated to play important roles in a number of biological systems including gene regulation and the inhibition of enzyme function. Recently, our laboratory reported on the
John B Sutherland et al.
Applied microbiology and biotechnology, 67(3), 405-411 (2005-04-28)
Cultures of Umbelopsis ramanniana (=Mucor ramannianus) were grown in fluid Sabouraud medium for 3 days, dosed with 0.23 mM benzo[f]quinoline, benzo[h]quinoline, or phenanthridine (benzo[c]quinoline), and incubated for another 18 days. Cultures were extracted and metabolites (66-75% of the UV absorbance)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service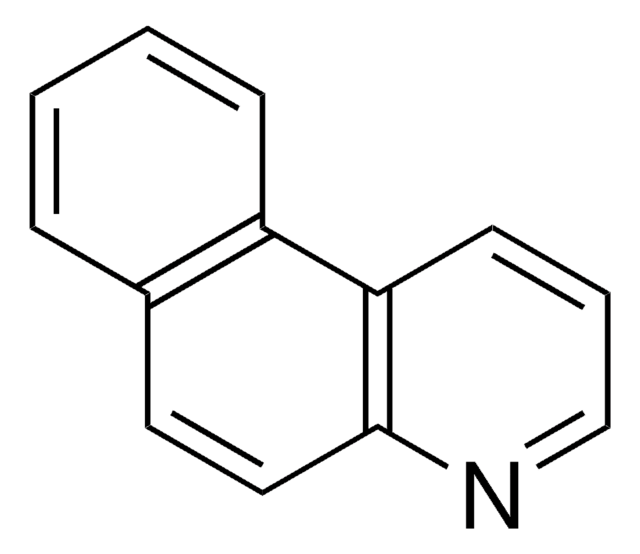










![Dibenz[c,h]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/364/643/698df9fb-5b7d-467a-b47e-c8318e2ed298/640/698df9fb-5b7d-467a-b47e-c8318e2ed298.png)
![Benz[g]isoquinoline-5,10-dione 99%](/deepweb/assets/sigmaaldrich/product/structures/484/029/288c4a9d-19c2-4b51-82c1-f43b50ea05b0/640/288c4a9d-19c2-4b51-82c1-f43b50ea05b0.png)