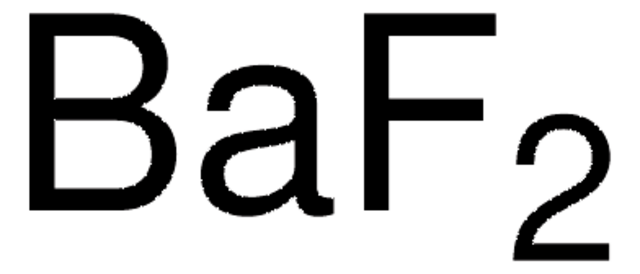All Photos(2)
About This Item
Empirical Formula (Hill Notation):
BaF2
CAS Number:
Molecular Weight:
175.32
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.55
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Assay
98%
form
powder
bp
2260 °C (lit.)
density
4.89 g/mL at 25 °C (lit.)
SMILES string
F[Ba]F
InChI
1S/Ba.2FH/h;2*1H/q+2;;/p-2
InChI key
OYLGJCQECKOTOL-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Features and Benefits
Primary glass modifier in fluorozirconate glasses.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and fluorescence of neodymium-doped barium fluoride nanoparticles.
Bender CM, et al.
Chemistry of Materials, 12(7), 1969-1976 (2000)
Large barium fluoride detectors.
Wisshak K and Kappeler F.
Nuclear Instruments & Methods in Physics Research. Section A, Accelerators, Spectrometers, Detectors and Associated Equipment, 227(1), 91-96 (1984)
Refractive properties of barium fluoride.
Mayakova MN, et al.
Journal of the Optical Society of America, 54(5), 628-630 (1964)
Jian Ruan et al.
Optics express, 17(7), 5163-5169 (2009-04-01)
Bi-doped BaF(2) crystal was grown by the temperature gradient technique and its spectral properties were investigated. The absorption, emission and excitation spectra were measured at room temperature. Two broadband emissions centered at 1070 and 1500 nm were observed in Bi-doped
S Tavernier et al.
Medical progress through technology, 17(3-4), 237-241 (1991-01-01)
The work presented is part of a design study for a Positron Emission Tomograph (PET) scanner based on the use of BaF2 scintillator and photosensitive wire chambers. The detection efficiency for gamma radiation of 511 keV is found close to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service