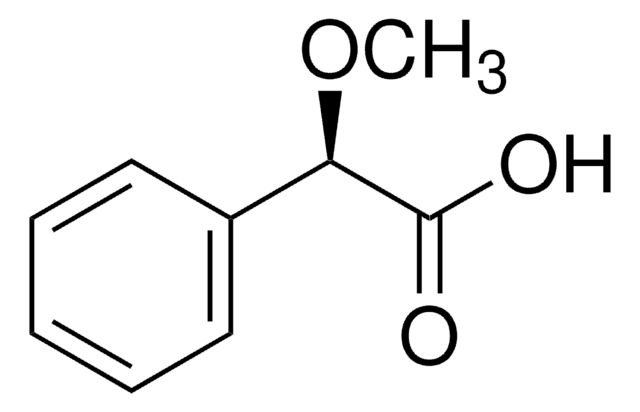
M2876
(±)-α-Methoxyphenylacetic acid
crystalline
Synonym(s):
O-Methyl-DL-mandelic acid, MOPA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(OCH3)COOH
CAS Number:
Molecular Weight:
166.17
Beilstein:
2209197
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
crystalline
Quality Level
bp
165 °C/18 mmHg (lit.)
mp
69-71 °C (lit.)
SMILES string
COC(C(O)=O)c1ccccc1
InChI
1S/C9H10O3/c1-12-8(9(10)11)7-5-3-2-4-6-7/h2-6,8H,1H3,(H,10,11)
InChI key
DIWVBIXQCNRCFE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(±)-α-Methoxyphenylacetic acid can be used as a reactant to synthesize:
- N
- -(Cyclohexylmethyl)-2-methoxy-2-phenylethylamine by reacting with cyclohexylmethylamine in the presence of EDCI/HOBt/Et3N.
- 4-(methoxyphenylmethyl)-2-methylpyridine by reacting with 2-methyl-4-pyridinecarbonitrile via decarboxylative arylation reaction in the presence of a photocatalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shoko Yamazaki et al.
The Journal of organic chemistry, 82(13), 6748-6763 (2017-06-13)
Catalytic cyclization of amides of ethenetricarboxylate bearing ether and acetal groups has been examined. The reaction of the amides bearing cyclic ether and acetal groups in the presence of Lewis acid such as Sc(OTf)3 gave spirocyclic piperidine derivatives as major
Yiyang Chen et al.
Organic letters, 21(7), 2130-2133 (2019-03-13)
1-(4-(9 H-Carbazol-9-yl)phenyl)-3-amino-9 H-fluorene-2,4-dicarbonitrile as a new photocatalyst for the decarboxylative cross-coupling reaction of α-amino acids or α-oxy carboxylic acids with arylnitriles is described. This light-driven reaction enables a variety of benzylic amines and ethers to be prepared from readily available
Bin Chang et al.
Journal of clinical microbiology, 53(10), 3318-3324 (2015-08-14)
Streptococcus pneumoniae colonizes the nasopharyngeal mucus in healthy people and causes otitis media, pneumonia, bacteremia, and meningitis. In this study, we analyzed an S. pneumoniae strain that caused 7 repeated pneumonia episodes in an 80-month-old patient with cerebral palsy during
Radoslaw Laufer et al.
Bioorganic & medicinal chemistry, 22(17), 4968-4997 (2014-07-22)
TTK kinase was identified by in-house siRNA screen and pursued as a tractable, novel target for cancer treatment. A screening campaign and systematic optimization, supported by computer modeling led to an indazole core with key sulfamoylphenyl and acetamido moieties at
Weidong Liu et al.
Chembiochem : a European journal of chemical biology, 16(6), 924-929 (2015-03-11)
A meso-diaminopimelate dehydrogenase (DAPDH) from Clostridium tetani E88 (CtDAPDH) was found to have low activity toward the D-amino acids other than its native substrate. Site-directed mutagenesis similar to that carried out on the residues mutated by Vedha-Peters et al. resulted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service