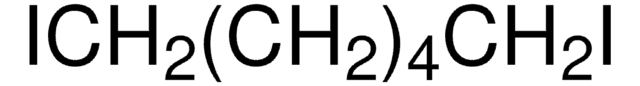D122602
1,4-Diiodobutane
≥99%, contains copper as stabilizer
Synonym(s):
Tetramethylene diiodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
I(CH2)4I
CAS Number:
Molecular Weight:
309.92
Beilstein:
1098276
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.621 (lit.)
bp
147-152 °C/26 mmHg (lit.)
mp
6 °C (lit.)
density
2.35 g/mL at 25 °C (lit.)
SMILES string
ICCCCI
InChI
1S/C4H8I2/c5-3-1-2-4-6/h1-4H2
InChI key
ROUYUBHVBIKMQO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jianghong Cai et al.
Molecules (Basel, Switzerland), 25(20) (2020-10-18)
In comparison with pristine sinomenine and carborane precursors, the calculations of molecular docking with matrix metalloproteinases (MMPs) and methylcarboranyl-n-butyl sinomenine showed improved interactions. Accordingly, methylcarboranyl-n-butyl sinomenine shows a high potential in the treatment of rheumatoid arthritis (RA) in the presence
Gas chromatographic determination of busulfan in plasma with electron-capture detection.
M Hassan et al.
Journal of chromatography, 277, 374-380 (1983-10-14)
D H Marchand et al.
Drug metabolism and disposition: the biological fate of chemicals, 16(1), 85-92 (1988-01-01)
gamma-Glutamyl-beta-(S-tetrahydrothiophenium)alanyl-glycine, the glutathione-sulfonium conjugate of busulfan and 1,4-diiodobutane, was identified in the bile of rats following intravenous administration of equimolar doses of either compound. The glutathione-sulfonium conjugate was synthesized from 1-bromo-4-chlorobutane and characterized by 1H and 13C NMR and FAB/MS.
D H Marchand et al.
Biochemical and biophysical research communications, 128(1), 360-367 (1985-04-16)
Rat liver glutathione S-transferases catalyzed the conjugation of 1,4-diiodobutane with glutathione in vitro. The reaction followed saturation kinetics and was dependent on the concentration of the enzyme, substrate and glutathione in the incubation media. S-Benzylglutathione inhibited the enzymatic conversion of
Ian C Watson et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(41), 9678-9690 (2019-05-16)
New N-heterocyclic olefins (NHOs) are described with functionalization on the ligand heterocyclic backbone and terminal alkylidene positions. Various PdII -NHO complexes have been formed and their use as pre-catalysts in Buchwald-Hartwig aminations was explored. The most active system for catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service