900555
(N-Isocyanoimino)triphenylphosphorane
95%
Synonym(s):
Pinc
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C19H15N2P
CAS Number:
Molecular Weight:
302.31
MDL number:
UNSPSC Code:
12352209
NACRES:
NA.22
Quality Level
Assay
95%
form
solid
application(s)
peptide synthesis
InChI
1S/C19H15N2P/c1-20-21-22(17-11-5-2-6-12-17,18-13-7-3-8-14-18)19-15-9-4-10-16-19/h2-16H
InChI key
NIDTXBFHPXMXTR-UHFFFAOYSA-N
General description
(N-Isocyanoimino)triphenylphosphorane (Pinc) is a bench-stable solid reagent widely used in organic synthesis. It is an amphoteric compound with unique properties that make it a versatile tool for various applications, including heterocycle synthesis and macrocyclization of peptides. Pinc facilitates cyclization and incorporation of a conformational control element into peptide macrocycles, leading to desired drug properties such as improved membrane permeability, lipophilicity, and aqueous solubility. Its ability to form oxadiazoles offers opportunities for the development of new transformations.


Application
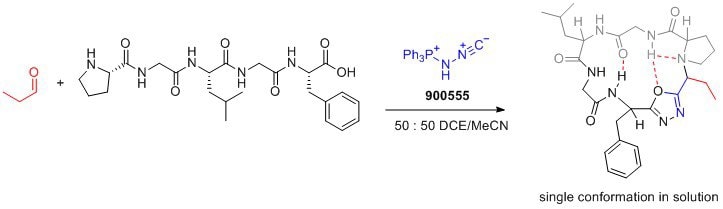
As reported by the lab of Andrei Yudin, Pinc enables zwitterionic-controlled generation of peptide macrocyles from linear peptides and aldehydes. It enables the formation of disubstituted 1,3,4-oxadiazoles by intercepting the key mixed anhydride characteristic of the Ugi reaction. Furthermore, the resulting peptide macrocycles containing oxadiazole exhibit improved drug properties such as cell-permeability and a rigid conformation.

Features and Benefits
- Bench-stable solid: Pinc is a stable reagent that can be easily handled and stored.
- Facilitates cyclization and incorporation of conformational control element: Pinc enables the formation of peptide macrocycles with a desired conformation, leading to improved drug properties.
- Amphoteric properties: Pinc′s amphoteric nature allows for the design of new multicomponent reactions, expanding its synthetic capabilities.
- Desired drug properties: The resulting peptide macrocycles exhibit enhanced membrane permeability, lipophilicity, and aqueous solubility, making them desirable for drug development.
- Versatile transformations: Pinc′s ability to form oxadiazoles opens up opportunities for the development of novel synthetic transformations.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service