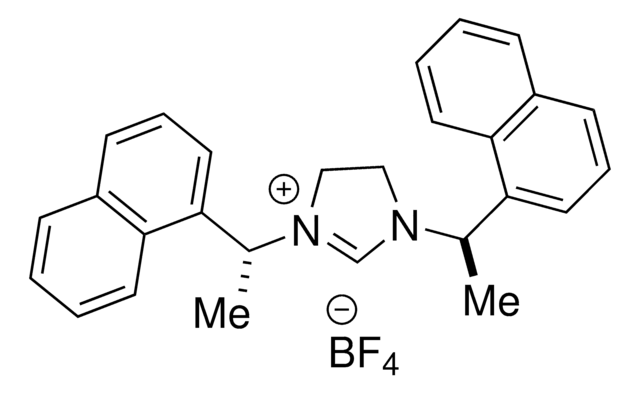
About This Item
Recommended Products
Assay
97%
form
powder
mp
228-232 °C
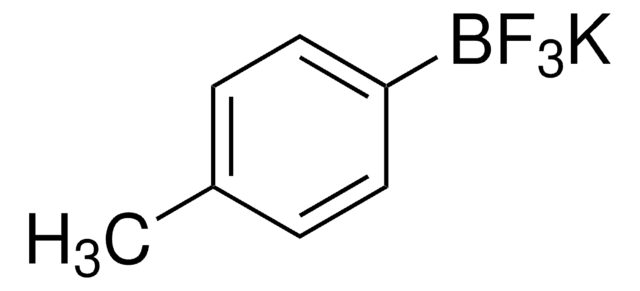
SMILES string
[K+].F[B-](F)(F)c1cccnc1
InChI
1S/C5H4BF3N.K/c7-6(8,9)5-2-1-3-10-4-5;/h1-4H;/q-1;+1
InChI key
MNCKCIOWIHHCSC-UHFFFAOYSA-N
Application
- Nickel-catalyzed cross-coupling reactions and C-O activation
- Suzuki cross-coupling
- Copper-catalyzed cross-coupling reactions
- Stereoselective Mukaiyama aldol reactions
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service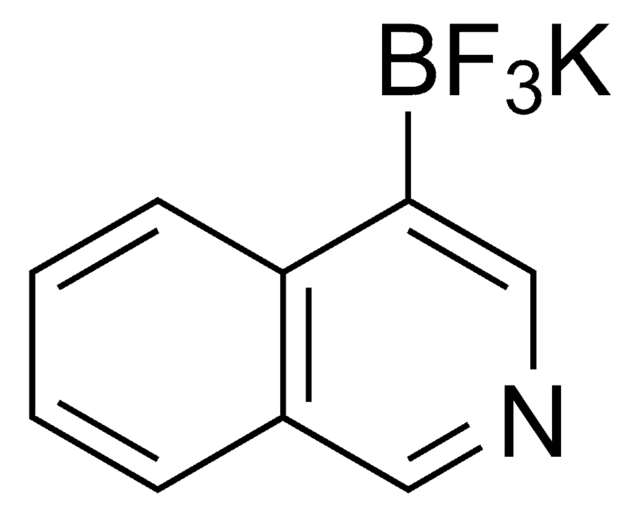

![Potassium trifluoro[(pyrrolidin-1-yl)methyl]borate AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/603/307/0f7c53df-8fc7-4d09-bc76-d55556863e97/640/0f7c53df-8fc7-4d09-bc76-d55556863e97.png)

![(5R,6S)-2-Mesityl-5,6-diphenyl-6,8-dihydro-5H-[1,2,4]triazolo[3,4-c][1,4]oxazin-2-ium tetrafluoroborate 97%](/deepweb/assets/sigmaaldrich/product/structures/219/182/9bfa803e-8970-4dd1-9cbf-9ebca2f74da2/640/9bfa803e-8970-4dd1-9cbf-9ebca2f74da2.png)