All Photos(1)
About This Item
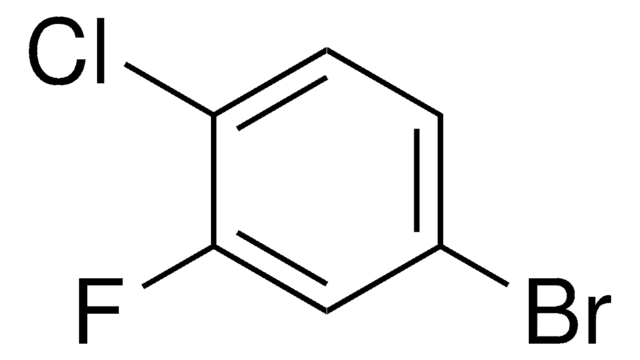
Linear Formula:
ClC6H3(F)I
CAS Number:
Molecular Weight:
256.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.604 (lit.)
bp
94-95 °C/15 mmHg (lit.)
density
2.008 g/mL at 25 °C (lit.)
functional group
chloro
fluoro
iodo
SMILES string
Fc1ccc(I)cc1Cl
InChI
1S/C6H3ClFI/c7-5-3-4(9)1-2-6(5)8/h1-3H
InChI key
OMASDGWBVAVFQZ-UHFFFAOYSA-N
General description
3-Chloro-4-fluoroiodobenzene, also known as 2-chloro-1-fluoro-4-iodobenzene [IUPAC name], is a polyhalobenzene. It participates in the synthesis of 3-carboxythiophene analogs.
Application
3-Chloro-4-fluoroiodobenzene may be used in the preparation of 2-chloro-1-(3,5-dimethoxyphenoxy)-4-iodobenzene by reacting with 3,5-dimethoxyphenol in the presence of cesium carbonate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fused thiophene derivatives as MEK inhibitors
Laing VE, et al.
Bioorganic & Medicinal Chemistry Letters, 22.1 , 472-475 (2012)
Selectivity issues in the catalytic multiphase reduction of functionalized halogenated aromatics over Pd/C, Pt/C, and Raney-Ni
Evdokimova, Galina, et al.
Applied Catalysis A: General, 271.1, 129-136 (2004)
Synthesis of Phloroglucinol Monoaryl Ethers
Sherwood AM, et al.
Synthesis, 44.08, 1208-1212 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service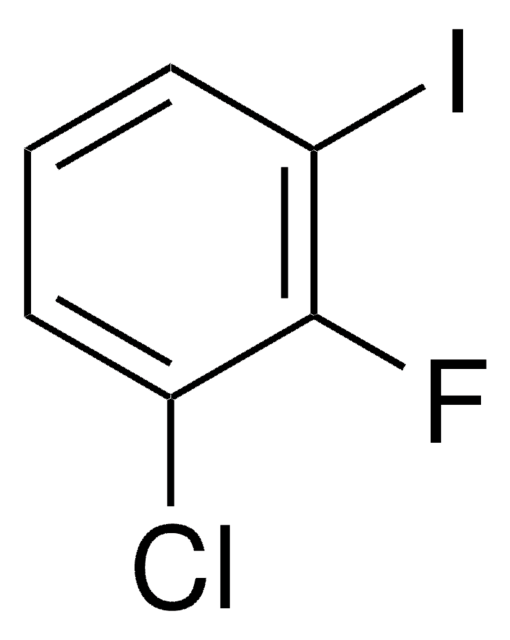


![6-Oxaspiro[2.5]octane-1-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/116/265/092e6253-e2e1-47f3-9513-19bf7d749365/640/092e6253-e2e1-47f3-9513-19bf7d749365.png)


